Dihydro-orotate dehydrogenase is physically associated with the respiratory complex and its loss leads to mitochondrial dysfunction
- PMID: 23216091
- PMCID: PMC3564035
- DOI: 10.1042/BSR20120097
Dihydro-orotate dehydrogenase is physically associated with the respiratory complex and its loss leads to mitochondrial dysfunction
Abstract
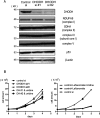
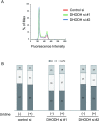
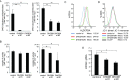
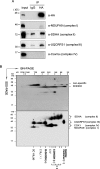
Some mutations of the DHODH (dihydro-orotate dehydrogenase) gene lead to postaxial acrofacial dysostosis or Miller syndrome. Only DHODH is localized at mitochondria among enzymes of the de novo pyrimidine biosynthesis pathway. Since the pyrimidine biosynthesis pathway is coupled to the mitochondrial RC (respiratory chain) via DHODH, impairment of DHODH should affect the RC function. To investigate this, we used siRNA (small interfering RNA)-mediated knockdown and observed that DHODH knockdown induced cell growth retardation because of G₂/M cell-cycle arrest, whereas pyrimidine deficiency usually causes G₁/S arrest. Inconsistent with this, the cell retardation was not rescued by exogenous uridine, which should bypass the DHODH reaction for pyrimidine synthesis. DHODH depletion partially inhibited the RC complex III, decreased the mitochondrial membrane potential, and increased the generation of ROS (reactive oxygen species). We observed that DHODH physically interacts with respiratory complexes II and III by IP (immunoprecipitation) and BN (blue native)/SDS/PAGE analysis. Considering that pyrimidine deficiency alone does not induce craniofacial dysmorphism, the DHODH mutations may contribute to the Miller syndrome in part through somehow altered mitochondrial function.
Figures
References
-
- McBride H. M., Neuspiel M., Wasiak S. Mitochondria: more than just a powerhouse. Curr. Biol. 2006;16:R551–R560. - PubMed
-
- DiMauro S., Schon E. A. Mitochondrial disorders in the nervous system. Annu. Rev. Neurosci. 2008;31:91–123. - PubMed
-
- Frenzel M., Rommelspacher H., Sugawa M. D., Dencher N. A. Ageing alters the supramolecular architecture of OxPhos complexes in rat brain cortex. Exp. Gerontol. 2010;45:563–572. - PubMed
-
- Wallace D. C. Mitochondrial DNA mutations in disease and aging. Environ. Mol. Mutagen. 2010;51:440–450. - PubMed
-
- Tuppen H. A., Blakely E. L., Turnbull D. M., Taylor R. W. Mitochondrial DNA mutations and human disease. Biochim. Biophys. Acta. 2010;1797:113–128. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

