Population genetic structure of the major malaria vector Anopheles funestus s.s. and allied species in southern Africa
- PMID: 23216696
- PMCID: PMC3533957
- DOI: 10.1186/1756-3305-5-283
Population genetic structure of the major malaria vector Anopheles funestus s.s. and allied species in southern Africa
Abstract
Background: Anopheles funestus s.s., one of the major malaria vectors in sub-Saharan Africa, belongs to a group of eleven African species that are morphologically similar at the adult stage, most of which do not transmit malaria. The population structure of An. funestus based on mitochondrial DNA data led to the description of two cryptic subdivisions, clade I widespread throughout Africa and clade II known only from Mozambique and Madagascar. In this study, we investigated five common members of the Anopheles funestus group in southern Africa in order to determine relationships within and between species.
Methods: A total of 155 specimens of An. funestus, An. parensis, An. vaneedeni, An. funestus-like and An. rivulorum from South Africa, Mozambique and Malawi were used for the study. The population genetic structure was assessed within and between populations using mitochondrial DNA.
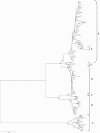
Results: The phylogenetic trees revealed three main lineages: 1) An. rivulorum; 2) An. funestus-like clade I and An. parensis clade II; and 3) An. funestus clades I and II, An. funestus-like clade II, An. parensis clade I and An. vaneedeni clades I and II. Within An. funestus, 32 specimens from Mozambique consisted of 40.6% clade I and 59.4% clade II while all 21 individuals from Malawi were clade I. In the analysis of mitochondrial DNA sequences, there were 37 polymorphic sites and 9 fixed different nucleotides for ND5 and 21 polymorphic sites and 6 fixed different nucleotides for COI between the two An. funestus clades. The results for COI supported the ND5 analysis.
Conclusion: This is the first report comparing An. funestus group species including An. funestus clades I and II and the new species An. funestus-like. Anopheles funestus clade I is separated from the rest of the members of the An. funestus subgroup and An. funestus-like is distinctly distributed from the other species in this study. However, there were two clades for An. funestus-like, An. parensis and An. vaneedeni. Further investigations are needed to determine what these results mean in terms of the specific status of the clades within each taxon and whether this has any epidemiological implications for malaria transmission.
Figures

References
-
- World Health Organization. The world malaria report 2011. Geneva: World Health Organization; 2011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

