The impact of physical activity on fatigue and quality of life in lung cancer patients: a randomised controlled trial protocol
- PMID: 23216897
- PMCID: PMC3534237
- DOI: 10.1186/1471-2407-12-572
The impact of physical activity on fatigue and quality of life in lung cancer patients: a randomised controlled trial protocol
Abstract
Background: People with lung cancer have substantial symptom burden and more unmet needs than the general cancer population. Physical activity (PA) has been shown to positively influence quality of life (QOL), fatigue and daily functioning in the curative treatment of people with breast and colorectal cancers and lung diseases, as well as in palliative settings. A randomised controlled trial (RCT) is needed to determine if lung cancer patients benefit from structured PA intervention. The Physical Activity in Lung Cancer (PAL) trial is designed to evaluate the impact of a 2-month PA intervention on fatigue and QOL in patients with non-resectable lung cancer. Biological mechanisms will also be studied.
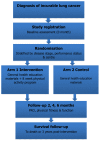
Methods/design: A multi-centre RCT with patients randomised to usual care or a 2-month PA programme, involving supervised PA sessions including a behavioural change component and home-based PA. QOL questionnaires, disease and functional status and body composition will be assessed at baseline, 2, 4 and 6 months follow-up. The primary endpoint is comparative levels of fatigue between the 2 arms. Secondary endpoints include: QOL, functional abilities and physical function. Exploratory endpoints include: anxiety, depression, distress, dyspnoea, PA behaviour, fitness, hospitalisations, survival, cytokines and insulin-like growth factor levels.
Discussion: This study will provide high-level evidence of the effect of PA programmes on cancer-related fatigue and QOL in patients with advanced lung cancer. If positive, the study has the potential to change care for people with cancer using a simple, inexpensive intervention to improve their QOL and help them maintain independent function for as long as possible.
Trial registration: Australian New Zealand Clinical Trials Registry No. ACTRN12609000971235.
Figures
References
-
- Society AC. Cancer facts & figures 2012. Atlanta: American Cancer Society; 2012.
-
- Gloeckler LA, Eisner MP. In: SEER Survival Monograph: Cancer Survival Among Adults: US SEER Program, 1988–2001, Patient and Tumor Characteristics. Volume NIH Pub. No. 07–6215. Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J, editor. Bethesda, MD: National Cancer Institute, SEER Program; 2007. Cancer of the lung.
-
- Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical