Low-molecular-weight thiol-dependent antioxidant and antinitrosative defences in Salmonella pathogenesis
- PMID: 23217033
- PMCID: PMC3885168
- DOI: 10.1111/mmi.12119
Low-molecular-weight thiol-dependent antioxidant and antinitrosative defences in Salmonella pathogenesis
Abstract
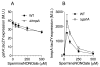
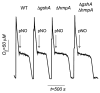
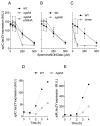
We found herein that the intracytoplasmic pool of the low-molecular-weight (LMW) thiol glutathione (GSH) is readily oxidized in Salmonella exposed to nitric oxide (NO). The hypersusceptibility of gshA and gshB mutants lacking γ-glutamylcysteine and glutathione synthetases to NO and S-nitrosoglutathione indicates that GSH antagonizes the bacteriostatic activity of reactive nitrogen species. Metabolites of the GSH biosynthetic pathway do not affect the enzymatic activity of classical NO targets such as quinol oxidases. In contrast, LMW thiols diminish the nitrosative stress experienced by enzymes, such as glutamine oxoglutarate amidotransferase, that contain redox active cysteines. LMW thiols also preserve the transcription of Salmonella pathogenicity island 2 gene targets from the inhibitory activity of nitrogen oxides. These findings are consistent with the idea that GSH scavenges reactive nitrogen species (RNS) other than NO. Compared with the adaptive response afforded by inducible systems such as the hmp-encoded flavohaemoprotein, gshA, encoding the first step of GSH biosynthesis, is constitutively expressed in Salmonella. An acute model of salmonellosis has revealed that the antioxidant and antinitrosative properties associated with the GSH biosynthetic pathway represent a first line of Salmonella resistance against reactive oxygen and nitrogen species engendered in the context of a functional NRAMP1(R) divalent metal transporter.
© 2012 Blackwell Publishing Ltd.
Conflict of interest statement
The authors have no conflict of interest.
Figures




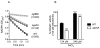
References
-
- Ables GP, Takamatsu D, Noma H, El-Shazly S, Jin HK, Taniguchi T, Sekikawa K, Watanabe T. The roles of Nramp1 and Tnfα genes in nitric oxide production and their effect on the growth of Salmonella typhimurium in macrophages from Nramp1 congenic and tumor necrosis factor-alpha−/− mice. J Interferon Cytokine Res. 2001;21:53–62. - PubMed
-
- Baker MA, Cerniglia GJ, Zaman A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem. 1990;190:360–365. - PubMed
-
- Bang IS, Liu L, Vazquez-Torres A, Crouch ML, Stamler JS, Fang FC. Maintenance of nitric oxide and redox homeostasis by the salmonella flavohemoglobin hmp. J Biol Chem. 2006;281:28039–28047. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

