Efficacy of artemether-lumefantrine as a treatment for uncomplicated Plasmodium vivax malaria in eastern Sudan
- PMID: 23217037
- PMCID: PMC3519545
- DOI: 10.1186/1475-2875-11-404
Efficacy of artemether-lumefantrine as a treatment for uncomplicated Plasmodium vivax malaria in eastern Sudan
Abstract
Background: Artemisinin-based combination therapy (ACT) is the treatment of choice for uncomplicated Plasmodium falciparum malaria in most areas of the world, where malaria is endemic, including Sudan. However, few published data are available on the use of ACT for treatment of P. vivax malaria.
Methods: This study was conducted at a health centre in Kassala, eastern Sudan, from October to December 2011. Patients with uncomplicated P. vivax malaria received artemether-lumefantrine (AL) tablets (containing 20mg artemether and 120 mg lumefantrine) and were monitored for 28 days.
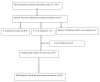
Results: Out of the 43 cases enrolled in this study, 38 completed the 28-day follow-up. Their mean age was 25.1 years (SD: 1.5). On day 3 following AL treatment, all of the patients were afebrile and aparasitaemic. By day 28, all 38 patients exhibited adequate clinical and parasitological responses to AL treatment. The cure rate was 100% and 88.4% for the per protocol analysis andfor the intention to treat analysis, respectively. Mild adverse effects (nausea, vomiting, abdominal pain, dizziness and/or rash) that resolved spontaneously were observed in four (10.5%) of the patients.
Conclusion: AL combination therapy was fully effective for treatment of P. vivax malaria in the study in eastern Sudan.
Trial registration: Trial. Gov: NCT01625871.
Figures
References
-
- Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR, Baird JK, Snow RW, Hay SI. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. - DOI - PMC - PubMed
-
- Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106. - PubMed
-
- Nigatu W, Abebe M, Dejene A. Plasmodium vivax and P. falciparum epidemiology in Gambella, south-west Ethiopia. Trop Med Parasitol. 1992;43:181–185. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical