Disruption of the endothelin A receptor in the nephron causes mild fluid volume expansion
- PMID: 23217151
- PMCID: PMC3537641
- DOI: 10.1186/1471-2369-13-166
Disruption of the endothelin A receptor in the nephron causes mild fluid volume expansion
Abstract
Background: Endothelin, via endothelin A receptors (ETA), exerts multiple pathologic effects that contribute to disease pathogenesis throughout the body. ETA antagonists ameliorate many experimental diseases and have been extensively utilized in clinical trials. The utility of ETA blockers has been greatly limited, however, by fluid retention, sometimes leading to heart failure or death. To begin to examine this issue, the effect of genetic disruption of ETA in the nephron on blood pressure and salt handling was determined.
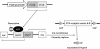
Methods: Mice were generated with doxycycline-inducible nephron-specific ETA deletion using Pax8-rtTA and LC-1 transgenes on the background of homozygous loxP-flanked ETA alleles. Arterial pressure, Na metabolism and measures of body fluid volume status (hematocrit and impedance plethysmography) were assessed.
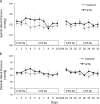
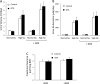
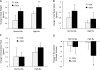
Results: Absence of nephron ETA did not alter arterial pressure whether mice were ingesting a normal or high Na diet. Nephron ETA disruption did not detectably affect 24 hr Na excretion or urine volume regardless of Na intake. However, mice with nephron ETA knockout that were fed a high Na diet had mild fluid retention as evidenced by an increase in body weight and a fall in hematocrit.
Conclusions: Genetic deletion of nephron ETA causes very modest fluid retention that does not alter arterial pressure. Nephron ETA, under normal conditions, likely do not play a major role in regulation of Na excretion or systemic hemodynamics.
Figures


References
-
- Battistini B, Berthiaume N, Kelland N, Webb D, Kohan D. Profile of past and current clinical trials involving endothelin receptor antagonists: the novel “-sentan” class of drug. Exp Biol Med. 2006;231:653–695. - PubMed
-
- Barton M, Kohan DE. Endothelin antagonists in clinical trials: lessons learned. Contrib Nephrol. 2011;172:255–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

