HTLV-3/4 and simian foamy retroviruses in humans: discovery, epidemiology, cross-species transmission and molecular virology
- PMID: 23217627
- PMCID: PMC7111966
- DOI: 10.1016/j.virol.2012.09.035
HTLV-3/4 and simian foamy retroviruses in humans: discovery, epidemiology, cross-species transmission and molecular virology
Abstract
Non-human primates are considered to be likely sources of viruses that can infect humans and thus pose a significant threat to human population. This is well illustrated by some retroviruses, as the simian immunodeficiency viruses and the simian T lymphotropic viruses, which have the ability to cross-species, adapt to a new host and sometimes spread. This leads to a pandemic situation for HIV-1 or an endemic one for HTLV-1. Here, we present the available data on the discovery, epidemiology, cross-species transmission and molecular virology of the recently discovered HTLV-3 and HTLV-4 deltaretroviruses, as well as the simian foamy retroviruses present in different human populations at risk, especially in central African hunters. We discuss also the natural history in humans of these retroviruses of zoonotic origin (magnitude and geographical distribution, possible inter-human transmission). In Central Africa, the increase of the bushmeat trade during the last decades has opened new possibilities for retroviral emergence in humans, especially in immuno-compromised persons.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
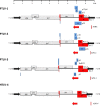
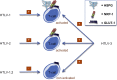

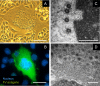

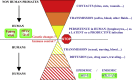
References
-
- Achong B.G., Mansell P.W., Epstein M.A. A new human virus in cultures from a nasopharyngeal carcinoma. J. Pathol. 1971;103:P18. - PubMed
-
- Aghokeng A.F., Liu W., Bibollet-Ruche F., Loul S., Mpoudi-Ngole E., Laurent C., Mwenda J.M., Langat D.K., Chege G.K., McClure H.M., Delaporte E., Shaw G.M., Hahn B.H., Peeters M. Widely varying SIV prevalence rates in naturally infected primate species from Cameroon. Virology. 2006;345:174–189. - PubMed
-
- Ahuka-Mundeke S., Mbala-Kingebeni P., Liegeois F., Ayouba A., Lunguya-Metila O., Demba D., Bilulu G., Mbenzo-Abokome V., Inogwabini B.I., Muyembe-Tamfum J.J., Delaporte E., Peeters M. Identification and molecular characterization of new simian T cell lymphotropic viruses in nonhuman primates bushmeat from the Democratic Republic of Congo. AIDS Res. Hum. Retroviruses. 2012;28:628–635. - PMC - PubMed
-
- Banerjee P., Feuer G., Barker E. Human T-cell leukemia virus type 1 (HTLV-1) p12I down-modulates ICAM-1 and −2 and reduces adherence of natural killer cells, thereby protecting HTLV-1-infected primary CD4+ T cells from autologous natural killer cell-mediated cytotoxicity despite the reduction of major histocompatibility complex class I molecules on infected cells. J. Virol. 2007;81:9707–9717. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

