The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism
- PMID: 23217706
- PMCID: PMC3526953
- DOI: 10.1016/j.cell.2012.10.047
The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism
Abstract
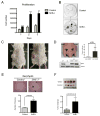
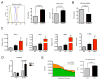
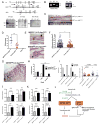
Reprogramming of cellular metabolism is a key event during tumorigenesis. Despite being known for decades (Warburg effect), the molecular mechanisms regulating this switch remained unexplored. Here, we identify SIRT6 as a tumor suppressor that regulates aerobic glycolysis in cancer cells. Importantly, loss of SIRT6 leads to tumor formation without activation of known oncogenes, whereas transformed SIRT6-deficient cells display increased glycolysis and tumor growth, suggesting that SIRT6 plays a role in both establishment and maintenance of cancer. By using a conditional SIRT6 allele, we show that SIRT6 deletion in vivo increases the number, size, and aggressiveness of tumors. SIRT6 also functions as a regulator of ribosome metabolism by corepressing MYC transcriptional activity. Lastly, Sirt6 is selectively downregulated in several human cancers, and expression levels of SIRT6 predict prognosis and tumor-free survival rates, highlighting SIRT6 as a critical modulator of cancer metabolism. Our studies reveal SIRT6 to be a potent tumor suppressor acting to suppress cancer metabolism.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




Comment in
-
SIRT6 puts cancer metabolism in the driver's seat.Cell. 2012 Dec 7;151(6):1155-6. doi: 10.1016/j.cell.2012.11.020. Cell. 2012. PMID: 23217699 Free PMC article.
References
-
- Anders M, Fehlker M, Wang Q, Wissmann C, Pilarsky C, Kemmner W, Hocker M. Microarray meta-analysis defines global angiogenesis-related gene expression signatures in human carcinomas. Mol Carcinog 2011 - PubMed
-
- Arabi A, Wu S, Ridderstrale K, Bierhoff H, Shiue C, Fatyol K, Fahlen S, Hydbring P, Soderberg O, Grummt I, et al. c-Myc associates with ribosomal DNA and activates RNA polymerase I transcription. Nat Cell Biol. 2005;7:303–310. - PubMed
-
- Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology. 2008;55:2016–2027. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM093072/GM/NIGMS NIH HHS/United States
- K08 EB012859/EB/NIBIB NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 CA175727/CA/NCI NIH HHS/United States
- P50 HG006193/HG/NHGRI NIH HHS/United States
- R01 GM101171/GM/NIGMS NIH HHS/United States
- P50HG006193/HG/NHGRI NIH HHS/United States
- P30 AG013283/AG/NIA NIH HHS/United States
- DK088190-01A1/DK/NIDDK NIH HHS/United States
- R01 DK088190/DK/NIDDK NIH HHS/United States
- GM101171/GM/NIGMS NIH HHS/United States
- GM093072-01/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

