TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells
- PMID: 23217710
- PMCID: PMC3522178
- DOI: 10.1016/j.cell.2012.10.041
TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells
Abstract
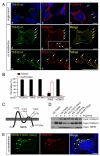
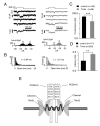
Hair cells are mechanosensors for the perception of sound, acceleration, and fluid motion. Mechanotransduction channels in hair cells are gated by tip links, which connect the stereocilia of a hair cell in the direction of their mechanical sensitivity. The molecular constituents of the mechanotransduction channels of hair cells are not known. Here, we show that mechanotransduction is impaired in mice lacking the tetraspan TMHS. TMHS binds to the tip-link component PCDH15 and regulates tip-link assembly, a process that is disrupted by deafness-causing Tmhs mutations. TMHS also regulates transducer channel conductance and is required for fast channel adaptation. TMHS therefore resembles other ion channel regulatory subunits such as the transmembrane alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor regulatory proteins (TARPs) of AMPA receptors that facilitate channel transport and regulate the properties of pore-forming channel subunits. We conclude that TMHS is an integral component of the hair cell's mechanotransduction machinery that functionally couples PCDH15 to the transduction channel.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures





References
-
- Adato A, Michel V, Kikkawa Y, Reiners J, Alagramam KN, Weil D, Yonekawa H, Wolfrum U, El-Amraoui A, Petit C. Interactions in the network of Usher syndrome type 1 proteins. Hum Mol Genet. 2005;14:347–356. - PubMed
-
- Alagramam KN, Murcia CL, Kwon HY, Pawlowski KS, Wright CG, Woychik RP. The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat Genet. 2001;27:99–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

