Single neuron activity and theta modulation in postrhinal cortex during visual object discrimination
- PMID: 23217745
- PMCID: PMC3523310
- DOI: 10.1016/j.neuron.2012.10.039
Single neuron activity and theta modulation in postrhinal cortex during visual object discrimination
Abstract
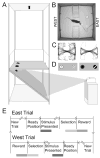
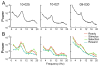
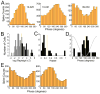
Postrhinal cortex, rodent homolog of the primate parahippocampal cortex, processes spatial and contextual information. Our hypothesis of postrhinal function is that it serves to encode context, in part, by forming representations that link objects to places. To test this hypothesis, we recorded postrhinal neurons and local field potentials (LFPs) in rats trained on a two-choice, visual discrimination task. As predicted, many postrhinal neurons signaled object-location conjunctions. Another large proportion encoded egocentric motor responses. In addition, postrhinal LFPs exhibited strong oscillatory rhythms in the theta band, and many postrhinal neurons were phase locked to theta. Although correlated with running speed, theta power was lower than predicted by speed alone immediately before and after choice. However, theta power was significantly increased following incorrect decisions, suggesting a role in signaling error. These findings provide evidence that postrhinal cortex encodes representations that link objects to places and suggest postrhinal theta modulation extends to cognitive as well as spatial functions.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures




References
-
- Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb Cortex. 2007;17:1493–1503. - PubMed
-
- Atienza M, Crespo-Garcia M, Cantero JL. Semantic congruence enhances memory of episodic associations: role of theta oscillations. J Cogn Neurosci. 2011;23:75–90. - PubMed
-
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

