Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice
- PMID: 23218253
- PMCID: PMC3570635
- DOI: 10.1016/j.biopsych.2012.09.026
Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice
Abstract
Background: Human immunodeficiency virus (HIV) associated neurocognitive disorders (HAND), including memory dysfunction, continue to be a major clinical manifestation of HIV type-1 infection. Viral proteins released by infected glia are thought to be the principal triggers of inflammation and bystander neuronal injury and death, thereby driving key symptomatology of HAND.
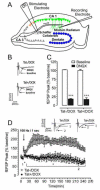
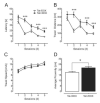
Methods: We used a glial fibrillary acidic protein-driven, doxycycline-inducible HIV type-1 transactivator of transcription (Tat) transgenic mouse model and examined structure-function relationships in hippocampal pyramidal cornu ammonis 1 (CA1) neurons using morphologic, electrophysiological (long-term potentiation [LTP]), and behavioral (Morris water maze, fear-conditioning) approaches.
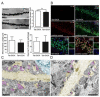
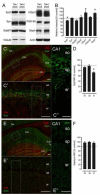
Results: Tat induction caused a variety of different inclusions in astrocytes characteristic of lysosomes, autophagic vacuoles, and lamellar bodies, which were typically present within distal cytoplasmic processes. In pyramidal CA1 neurons, Tat induction reduced the number of apical dendritic spines, while disrupting the distribution of synaptic proteins (synaptotagmin 2 and gephyrin) associated with inhibitory transmission but with minimal dendritic pathology and no evidence of pyramidal neuron death. Electrophysiological assessment of excitatory postsynaptic field potential at Schaffer collateral/commissural fiber-CA1 synapses showed near total suppression of LTP in mice expressing Tat. The loss in LTP coincided with disruptions in learning and memory.
Conclusions: Tat expression in the brain results in profound functional changes in synaptic physiology and in behavior that are accompanied by only modest structural changes and minimal pathology. Tat likely contributes to HAND by causing molecular changes that disrupt synaptic organization, with inhibitory presynaptic terminals containing synaptotagmin 2 appearing especially vulnerable.
Published by Elsevier Inc.
Figures

Similar articles
-
HIV-1 Tat inhibits long-term potentiation and attenuates spatial learning [corrected].Ann Neurol. 2004 Mar;55(3):362-71. doi: 10.1002/ana.10844. Ann Neurol. 2004. PMID: 14991814
-
Methamphetamine augment HIV-1 Tat mediated memory deficits by altering the expression of synaptic proteins and neurotrophic factors.Brain Behav Immun. 2018 Jul;71:37-51. doi: 10.1016/j.bbi.2018.04.018. Epub 2018 May 2. Brain Behav Immun. 2018. PMID: 29729322 Free PMC article.
-
HIV-1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron subpopulations.J Neurovirol. 2016 Dec;22(6):747-762. doi: 10.1007/s13365-016-0447-2. Epub 2016 May 13. J Neurovirol. 2016. PMID: 27178324 Free PMC article.
-
Hormonal regulation of hippocampal dendritic morphology and synaptic plasticity.Cell Adh Migr. 2008 Oct-Dec;2(4):269-75. doi: 10.4161/cam.2.4.6354. Epub 2008 Oct 28. Cell Adh Migr. 2008. PMID: 19262152 Free PMC article. Review.
-
The importance of considering all attributes of memory in behavioral endophenotyping of mouse models of genetic disease.Behav Neurosci. 2012 Jun;126(3):371-80. doi: 10.1037/a0028453. Behav Neurosci. 2012. PMID: 22642882 Free PMC article. Review.
Cited by
-
Glycogen synthase kinase-3 inhibition rescues sex-dependent contextual fear memory deficit in human immunodeficiency virus-1 transgenic mice.Br J Pharmacol. 2020 Dec;177(24):5658-5676. doi: 10.1111/bph.15288. Epub 2020 Nov 23. Br J Pharmacol. 2020. PMID: 33080056 Free PMC article.
-
Chronic HIV-1 Tat exposure alters anterior cingulate cortico-basal ganglia-thalamocortical synaptic circuitry, associated behavioral control, and immune regulation in male mice.Brain Behav Immun Health. 2020 May;5:100077. doi: 10.1016/j.bbih.2020.100077. Epub 2020 Apr 29. Brain Behav Immun Health. 2020. PMID: 33083793 Free PMC article.
-
HIV-1 Tat-induced changes in synaptically-driven network activity adapt during prolonged exposure.Curr HIV Res. 2014;12(6):406-14. doi: 10.2174/1570162x13666150121110402. Curr HIV Res. 2014. PMID: 25613136 Free PMC article.
-
Inhibitory Neurotransmission Is Sex-Dependently Affected by Tat Expression in Transgenic Mice and Suppressed by the Fatty Acid Amide Hydrolase Enzyme Inhibitor PF3845 via Cannabinoid Type-1 Receptor Mechanisms.Cells. 2022 Mar 2;11(5):857. doi: 10.3390/cells11050857. Cells. 2022. PMID: 35269478 Free PMC article.
-
The HIV-1 transgenic rat model of neuroHIV.Brain Behav Immun. 2015 Aug;48:336-49. doi: 10.1016/j.bbi.2015.02.020. Epub 2015 Feb 27. Brain Behav Immun. 2015. PMID: 25733103 Free PMC article. Review.
References
-
- Hebb DO. The organization of behavior. Wiley & Sons; New York: 1949.
-
- Dayhoff JE. Computational properties of networks of synchronous groups of spiking neurons. Neural Comput. 2007;19:2433–2467. - PubMed
-
- Globus A, Scheibel AB. Loss of dendrite spines as an index of pre-synaptic terminal patterns. Nature. 1966;212:463–465. - PubMed
-
- Kruman I, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. - PubMed
-
- Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

