The action of selective CRAC channel blockers is affected by the Orai pore geometry
- PMID: 23218667
- PMCID: PMC3580291
- DOI: 10.1016/j.ceca.2012.11.005
The action of selective CRAC channel blockers is affected by the Orai pore geometry
Abstract
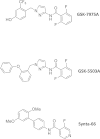
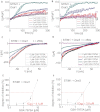
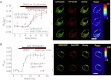
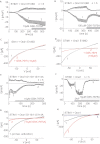
As the molecular composition of calcium-release activated calcium (CRAC) channels has been unknown for two decades, elucidation of selective inhibitors has been considerably hampered. By the identification of the two key components of CRAC channels, STIM1 and Orai1 have emerged as promising targets for CRAC blockers. The aim of this study was to thoroughly characterize the effects of two selective CRAC channel blockers on currents derived from STIM1/Orai heterologoulsy expressed in HEK293 cells. The novel compounds GSK-7975A and GSK-5503A were tested for effects on STIM1 mediated Orai1 or Orai3 currents by whole-cell patch-clamp recordings and for the effects on STIM1 oligomerisation or STIM1/Orai coupling by FRET microscopy. To investigate their site of action, inhibitory effects of these molecules were explored using Orai pore mutants. The GSK blockers inhibited Orai1 and Orai3 currents with an IC(50) of approximately 4μM and exhibited a substantially slower rate of onset than the typical pore blocker La(3+), together with almost no current recovery upon wash-out over 4min. For the less Ca(2+)-selective Orai1 E106D pore mutant, I(CRAC) inhibition was significantly reduced. FRET experiments indicated that neither STIM1-STIM1 oligomerization nor STIM1-Orai1 coupling was affected by these compounds. These CRAC channel blockers are acting downstream of STIM1 oligomerization and STIM1/Orai1 interaction, potentially via an allosteric effect on the selectivity filter of Orai. The elucidation of these CRAC current blockers represents a significant step toward the identification of CRAC channel-selective drug compounds.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures






Similar articles
-
Store-Independent Orai Channels Regulated by STIM.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review.
-
Authentic CRAC channel activity requires STIM1 and the conserved portion of the Orai N terminus.J Biol Chem. 2018 Jan 26;293(4):1259-1270. doi: 10.1074/jbc.M117.812206. Epub 2017 Dec 13. J Biol Chem. 2018. PMID: 29237734 Free PMC article.
-
Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels.J Biol Chem. 2007 Jun 15;282(24):17548-56. doi: 10.1074/jbc.M611374200. Epub 2007 Apr 23. J Biol Chem. 2007. PMID: 17452328
-
A Ca2(+ )release-activated Ca2(+) (CRAC) modulatory domain (CMD) within STIM1 mediates fast Ca2(+)-dependent inactivation of ORAI1 channels.J Biol Chem. 2009 Sep 11;284(37):24933-8. doi: 10.1074/jbc.C109.024083. Epub 2009 Jul 21. J Biol Chem. 2009. PMID: 19622747 Free PMC article.
-
The STIM1: Orai Interaction.Adv Exp Med Biol. 2016;898:25-46. doi: 10.1007/978-3-319-26974-0_2. Adv Exp Med Biol. 2016. PMID: 27161223 Review.
Cited by
-
Ion channels in innate and adaptive immunity.Annu Rev Immunol. 2015;33:291-353. doi: 10.1146/annurev-immunol-032414-112212. Annu Rev Immunol. 2015. PMID: 25861976 Free PMC article. Review.
-
Molecular pharmacology of store-operated CRAC channels.Channels (Austin). 2013 Sep-Oct;7(5):402-14. doi: 10.4161/chan.25292. Epub 2013 Aug 26. Channels (Austin). 2013. PMID: 23807116 Free PMC article. Review.
-
STIM1 and ORAI1 form a novel cold transduction mechanism in sensory and sympathetic neurons.EMBO J. 2023 Feb 1;42(3):e111348. doi: 10.15252/embj.2022111348. Epub 2022 Dec 16. EMBO J. 2023. PMID: 36524441 Free PMC article.
-
ORAI1 Ca2+ Channel as a Therapeutic Target in Pathological Vascular Remodelling.Front Cell Dev Biol. 2021 Apr 6;9:653812. doi: 10.3389/fcell.2021.653812. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33937254 Free PMC article. Review.
-
Photopharmacological modulation of native CRAC channels using azoboronate photoswitches.Proc Natl Acad Sci U S A. 2022 Mar 29;119(13):e2118160119. doi: 10.1073/pnas.2118160119. Epub 2022 Mar 21. Proc Natl Acad Sci U S A. 2022. PMID: 35312368 Free PMC article.
References
-
- Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: dynamics, homeostasis and remodelling. Nature Reviews Molecular Cell Biology. 2003;4(7):517–529. - PubMed
-
- Fischer B.S., Qin D., Kim K., McDonald T.V. Capsaicin inhibits Jurkat T-cell activation by blocking calcium entry current I(CRAC) Journal of Pharmacology and Experimental Therapeutics. 2001;299(1):238–246. - PubMed
-
- Gericke M., Oike M., Droogmans G., Nilius B. Inhibition of capacitative Ca2+ entry by a Cl− channel blocker in human endothelial cells. European Journal of Pharmacology. 1994;269(3):381–384. - PubMed
-
- Li J.H., Spence K.T., Dargis P.G., Christian E.P. Properties of Ca(2+) release-activated Ca(2+) channel block by 5-nitro-2-(3-phenylpropylamino)-benzoic acid in Jurkat cells. European Journal of Pharmacology. 2000;394(2–3):171–179. - PubMed
-
- Reinsprecht M., Rohn M.H., Spadinger R.J., Pecht I., Schindler H., Romanin C. Blockade of capacitive Ca2+ influx by Cl- channel blockers inhibits secretion from rat mucosal-type mast cells. Molecular Pharmacology. 1995;47(5):1014–1020. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

