Inhibition of norovirus 3CL protease by bisulfite adducts of transition state inhibitors
- PMID: 23218713
- PMCID: PMC3586229
- DOI: 10.1016/j.bmcl.2012.11.026
Inhibition of norovirus 3CL protease by bisulfite adducts of transition state inhibitors
Abstract
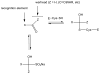
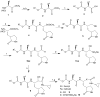
Noroviruses are the most common cause of acute viral gastroenteritis, accounting for >21 million cases annually in the US alone. Norovirus infections constitute an important health problem for which there are no specific antiviral therapeutics or vaccines. In this study, a series of bisulfite adducts derived from representative transition state inhibitors (dipeptidyl aldehydes and α-ketoamides) was synthesized and shown to exhibit anti-norovirus activity in a cell-based replicon system. The ED(50) of the most effective inhibitor was 60 nM. This study demonstrates for the first time the utilization of bisulfite adducts of transition state inhibitors in the inhibition of norovirus 3C-like protease in vitro and in a cell-based replicon system. The approach described herein can be extended to the synthesis of the bisulfite adducts of other classes of transition state inhibitors of serine and cysteine proteases, such as α-ketoheterocycles and α-ketoesters.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Structure-guided design and optimization of dipeptidyl inhibitors of norovirus 3CL protease. Structure-activity relationships and biochemical, X-ray crystallographic, cell-based, and in vivo studies.J Med Chem. 2015 Apr 9;58(7):3144-55. doi: 10.1021/jm5019934. Epub 2015 Mar 19. J Med Chem. 2015. PMID: 25761614 Free PMC article.
-
Structure-Guided Optimization of Dipeptidyl Inhibitors of Norovirus 3CL Protease.J Med Chem. 2020 Oct 22;63(20):11945-11963. doi: 10.1021/acs.jmedchem.0c01252. Epub 2020 Oct 2. J Med Chem. 2020. PMID: 32945669
-
Oxadiazole-Based Cell Permeable Macrocyclic Transition State Inhibitors of Norovirus 3CL Protease.J Med Chem. 2016 Mar 10;59(5):1899-913. doi: 10.1021/acs.jmedchem.5b01464. Epub 2016 Feb 8. J Med Chem. 2016. PMID: 26823007 Free PMC article.
-
Antiviral Drug Discovery: Norovirus Proteases and Development of Inhibitors.Viruses. 2019 Feb 25;11(2):197. doi: 10.3390/v11020197. Viruses. 2019. PMID: 30823509 Free PMC article. Review.
-
Norovirus Protease Structure and Antivirals Development.Viruses. 2021 Oct 14;13(10):2069. doi: 10.3390/v13102069. Viruses. 2021. PMID: 34696498 Free PMC article. Review.
Cited by
-
Recent Advances in the Discovery of Norovirus Therapeutics.J Med Chem. 2015 Dec 24;58(24):9438-50. doi: 10.1021/acs.jmedchem.5b00762. Epub 2015 Aug 17. J Med Chem. 2015. PMID: 26258852 Free PMC article. Review.
-
Potent inhibition of norovirus by dipeptidyl α-hydroxyphosphonate transition state mimics.Bioorg Med Chem Lett. 2013 Nov 1;23(21):5941-4. doi: 10.1016/j.bmcl.2013.08.073. Epub 2013 Aug 22. Bioorg Med Chem Lett. 2013. PMID: 24054123 Free PMC article.
-
Potent Protease Inhibitors of Highly Pathogenic Lagoviruses: Rabbit Hemorrhagic Disease Virus and European Brown Hare Syndrome Virus.Microbiol Spectr. 2022 Aug 31;10(4):e0014222. doi: 10.1128/spectrum.00142-22. Epub 2022 Jun 29. Microbiol Spectr. 2022. PMID: 35766511 Free PMC article.
-
Structural and inhibitor studies of norovirus 3C-like proteases.Virus Res. 2013 Dec 26;178(2):437-44. doi: 10.1016/j.virusres.2013.09.008. Epub 2013 Sep 17. Virus Res. 2013. PMID: 24055466 Free PMC article.
-
Structure-guided design and optimization of dipeptidyl inhibitors of norovirus 3CL protease. Structure-activity relationships and biochemical, X-ray crystallographic, cell-based, and in vivo studies.J Med Chem. 2015 Apr 9;58(7):3144-55. doi: 10.1021/jm5019934. Epub 2015 Mar 19. J Med Chem. 2015. PMID: 25761614 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

