Opioid receptors: distinct roles in mood disorders
- PMID: 23219016
- PMCID: PMC3594542
- DOI: 10.1016/j.tins.2012.11.002
Opioid receptors: distinct roles in mood disorders
Abstract
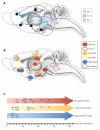
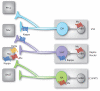
The roles of opioid receptors in pain and addiction have been extensively studied, but their function in mood disorders has received less attention. Accumulating evidence from animal research reveals that mu, delta and kappa opioid receptors (MORs, DORs and KORs, respectively) exert highly distinct controls over mood-related processes. DOR agonists and KOR antagonists have promising antidepressant potential, whereas the risk-benefit ratio of currently available MOR agonists as antidepressants remains difficult to evaluate, in addition to their inherent abuse liability. To date, both human and animal studies have mainly examined MORs in the etiology of depressive disorders, and future studies will address DOR and KOR function in established and emerging neurobiological aspects of depression, including neurogenesis, neurodevelopment, and social behaviors.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

References
-
- Bodnar RJ. Endogenous opiates and behavior: 2010. Peptides. 2011;32:2522–2552. - PubMed
-
- Berrocoso E, et al. Opiates as antidepressants. Curr Pharm Des. 2009;15:1612–1622. - PubMed
-
- Mueller TI, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156:1000–1006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

