Estradiol accelerates the effects of fluoxetine on serotonin 1A receptor signaling
- PMID: 23219224
- PMCID: PMC3610798
- DOI: 10.1016/j.psyneuen.2012.11.005
Estradiol accelerates the effects of fluoxetine on serotonin 1A receptor signaling
Abstract
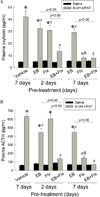
A major problem with current anti-depressant therapy is that it takes on average 6-7 weeks for remission. Since desensitization of serotonin (5-HT)1A receptor signaling contributes to the anti-depressive response, acceleration of the desensitization may reduce this delay in response to antidepressants. The purpose of the present study was to test the hypothesis that estradiol accelerates fluoxetine-induced desensitization of 5-HT1A receptor signaling in the paraventricular nucleus of the hypothalamus (PVN) of rats, via alterations in components of the 5-HT1A receptor signaling pathway. Ovariectomized rats were injected with estradiol and/or fluoxetine, then adrenocorticotropic hormone (ACTH) and oxytocin responses to a 5-HT1A receptor agonist (+)-8-hydroxy-2-dipropylaminotetralin (8-OH-DPAT) were examined to assess the function of 5-HT1A receptors in the PVN. Treatment with estradiol for either 2 or 7 days or fluoxetine for 2 days produced at most a partial desensitization of 5-HT1A receptor signaling, whereas 7 days of fluoxetine produced full desensitization. Combined treatment with estradiol and fluoxetine for 2 days produced nearly a full desensitization, demonstrating an accelerated response compared to either treatment alone. With two days of combined treatments, estradiol prevented the fluoxetine-induced increase in 5-HT1A receptor protein, which could contribute to the more rapid desensitization. Furthermore, EB treatment for 2 days decreased the abundance of the 35 kD Gαz protein which could contribute to the desensitization response. We found two isoforms of Gαz proteins with molecular mass of 35 and 33 kD, which differentially distributed in the detergent resistant microdomain (DRM) and in Triton X-100 soluble membrane region, respectively. The 35 kD Gαz proteins in the DRM can be sumoylated by SUMO1. Stimulation of 5-HT1A receptors with 8-OH-DPAT increases the sumoylation of Gαz proteins and reduces the 33 kD Gαz proteins, suggesting that these responses may be related to the desensitization of 5-HT1A receptors. Treatment with estradiol for 2 days also reduced the levels of the G-protein coupled estrogen receptor GPR30, possibly limiting to the ability of estradiol to produce only a partial desensitization response. These data provide evidence that estradiol may be effective as a short-term adjuvant to SSRIs to accelerate the onset of therapeutic effects.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no actual or potential financial and other conflicts of interest related to the submitted manuscript.
Figures




Similar articles
-
Estradiol potentiates 8-OH-DPAT-induced sumoylation of 5-HT₁A receptor: characterization and subcellular distribution of sumoylated 5-HT₁A receptors.Psychoneuroendocrinology. 2013 Nov;38(11):2542-53. doi: 10.1016/j.psyneuen.2013.05.016. Epub 2013 Jun 18. Psychoneuroendocrinology. 2013. PMID: 23786880 Free PMC article.
-
Estradiol induces partial desensitization of serotonin 1A receptor signaling in the paraventricular nucleus of the hypothalamus and alters expression and interaction of RGSZ1 and Gαz.Neuropharmacology. 2012 Apr;62(5-6):2040-9. doi: 10.1016/j.neuropharm.2012.01.001. Epub 2012 Jan 10. Neuropharmacology. 2012. PMID: 22251927 Free PMC article.
-
Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus.Neuroscience. 2009 Feb 18;158(4):1599-607. doi: 10.1016/j.neuroscience.2008.11.028. Epub 2008 Nov 21. Neuroscience. 2009. PMID: 19095043 Free PMC article.
-
Effects of the antidepressant fluoxetine on the subcellular localization of 5-HT1A receptors and SERT.Philos Trans R Soc Lond B Biol Sci. 2012 Sep 5;367(1601):2416-25. doi: 10.1098/rstb.2011.0361. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 22826342 Free PMC article. Review.
-
[CROSS-TALK BETWEEN 5-HT1A AND 5-HT7 RECEPTORS: ROLE IN THE AUTOREGULATION OF THE BRAIN SEROTONIN SYSTEM AND IN MECHANISM OF ANTIDEPRESSANTS ACTION].Ross Fiziol Zh Im I M Sechenova. 2015 Nov;101(11):1270-8. Ross Fiziol Zh Im I M Sechenova. 2015. PMID: 26995955 Review. Russian.
Cited by
-
Transcriptome Analysis on Maternal Separation Rats With Depression-Related Manifestations Ameliorated by Electroacupuncture.Front Neurosci. 2019 Apr 5;13:314. doi: 10.3389/fnins.2019.00314. eCollection 2019. Front Neurosci. 2019. PMID: 31024237 Free PMC article.
-
Female sexual function in different phenotypes of polycystic ovarian syndrome: a comparative cross-sectional study.Sci Rep. 2022 Nov 11;12(1):19317. doi: 10.1038/s41598-022-24026-7. Sci Rep. 2022. PMID: 36369524 Free PMC article.
-
Differential Effects of the G-Protein-Coupled Estrogen Receptor (GPER) on Rat Embryonic (E18) Hippocampal and Cortical Neurons.eNeuro. 2022 Jul 15;9(4):ENEURO.0475-21.2022. doi: 10.1523/ENEURO.0475-21.2022. Print 2022 Jul-Aug. eNeuro. 2022. PMID: 35788105 Free PMC article.
-
Regulation of Serotonin 1A Receptor SUMOylation by SENP2 and PIASxα.Int J Mol Sci. 2021 Dec 7;22(24):13176. doi: 10.3390/ijms222413176. Int J Mol Sci. 2021. PMID: 34947973 Free PMC article.
-
5-HT2A receptor loss does not alter acute fluoxetine-induced anxiety and exhibit sex-dependent regulation of cortical immediate early gene expression.Neuronal Signal. 2019 Feb 1;3(1):NS20180205. doi: 10.1042/NS20180205. eCollection 2019 Mar. Neuronal Signal. 2019. PMID: 32714597 Free PMC article.
References
-
- Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: Guilt by association. Neuroscientist. 2004;10:575–593. - PubMed
-
- Alexander JL, Dennerstein L, Woods NF, Kotz K, Halbreich U, Burt V, Richardson G. Neurobehavioral impact of menopause on mood. Expert. Rev. Neurother. 2007;7:S81–S91. - PubMed
-
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. - PubMed
-
- Artigas F, Adell A, Celada P. Pindolol augmentation of antidepressant response. Curr. Drug Targets. 2006;7:139–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

