Cell-to-cell transmission of viruses
- PMID: 23219376
- PMCID: PMC3587356
- DOI: 10.1016/j.coviro.2012.11.004
Cell-to-cell transmission of viruses
Abstract
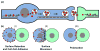
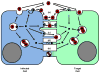
The life cycle of most viruses involves the release of particles into the extracellular space. Consequently, the study of virus egress as well as virus entry has focused almost exclusively on the biology of cell-free virus. However, cell-free virus spread is often very inefficient. Specific barriers, either located in the donor cell or in the target cell, prevent efficient spread by the cell-free mode. In contrast, viral spread by direct cell-cell contact is largely unaffected by most of these barriers resulting in preferential spread by cell-to-cell transmission. Virus cell-to-cell transmission allows an efficient coordination of several steps of the viral life cycle. It often involves complex inter-cellular adhesion, cellular polarity and intra-cellular trafficking. Because virus cell-to-cell transmission can involve transmission through zones of tight cell-cell contact that are resistant to neutralizing antibodies and reach a high local particle concentration, cell-to-cell transmission can contribute to the pathogenesis of viral infections.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

