NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells
- PMID: 23219391
- PMCID: PMC4275304
- DOI: 10.1016/j.immuni.2012.08.027
NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells
Abstract
Cytopenias are key prognostic indicators of life-threatening infection, contributing to immunosuppression and mortality. Here we define a role for Caspase-1-dependent death, known as pyroptosis, in infection-induced cytopenias by studying inflammasome activation in hematopoietic progenitor cells. The NLRP1a inflammasome is expressed in hematopoietic progenitor cells and its activation triggers their pyroptotic death. Active NLRP1a induced a lethal systemic inflammatory disease that was driven by Caspase-1 and IL-1β but was independent of apoptosis-associated speck-like protein containing a CARD (ASC) and ameliorated by IL-18. Surprisingly, in the absence of IL-1β-driven inflammation, active NLRP1a triggered pyroptosis of hematopoietic progenitor cells resulting in leukopenia at steady state. During periods of hematopoietic stress induced by chemotherapy or lymphocytic choriomeningitis virus (LCMV) infection, active NLRP1a caused prolonged cytopenia, bone marrow hypoplasia, and immunosuppression. Conversely, NLRP1-deficient mice showed enhanced recovery from chemotherapy and LCMV infection, demonstrating that NLRP1 acts as a cellular sentinel to alert Caspase-1 to hematopoietic and infectious stress.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
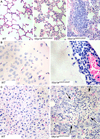
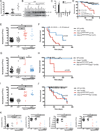
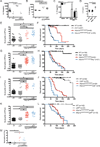


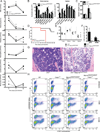
Comment in
-
NLRP1 joins the dark side?Immunity. 2012 Dec 14;37(6):950-2. doi: 10.1016/j.immuni.2012.11.008. Immunity. 2012. PMID: 23244714
References
-
- Arber C, BitMansour A, Sparer TE, Higgins JP, Mocarski ES, Weissman IL, Shizuru JA, Brown JM. Common lymphoid progenitors rapidly engraft and protect against lethal murine cytomegalovirus infection after hematopoietic stem cell transplantation. Blood. 2003;102:421–428. - PubMed
-
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. - PubMed
-
- Battegay M, Cooper S, Althage A, Banziger J, Hengartner H, Zinkernagel RM. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J Virol Methods. 1991;33:191–198. - PubMed
-
- Bendahmane A, Farnham G, Moffett P, Baulcombe DC. Constitutive gain-of-function mutants in a nucleotide binding site-leucine rich repeat protein encoded at the Rx locus of potato. Plant J. 2002;32:195–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

