Interaction of cepharanthine with immobilized heat shock protein 90α (Hsp90α) and screening of Hsp90α inhibitors
- PMID: 23219559
- PMCID: PMC4632246
- DOI: 10.1016/j.ab.2012.11.010
Interaction of cepharanthine with immobilized heat shock protein 90α (Hsp90α) and screening of Hsp90α inhibitors
Erratum in
- Anal Biochem. 2013 May 15;436(2):65
Abstract
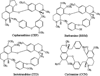
Heat shock protein 90α (Hsp90α) immobilized on aminopropyl silica gels was prepared via the N- or C-terminal, which was termed Hsp90α-NT or Hsp90α-CT, respectively. Binding interactions of biscoclaurine alkaloids (cepharanthine (CEP), berbamine (BBM), isotetrandrine (ITD), and cycleanine (CCN)) with Hsp90α were examined using the Hsp90α-NT or -CT columns by frontal and zonal chromatography studies. The dissociation constants of CEP, BBM, ITD, and CCN to Hsp90α-NT were estimated to be 5.3, 18.6, 46.3, and 159 μM, respectively, by frontal chromatography techniques. Similar results were obtained with the Hsp90α-CT column. These data suggest that these biscoclaurine alkaloids interact with the middle domain of Hsp90α. This was confirmed by demonstrating that CEP competed with endothelial nitric oxide synthase at the middle domain of Hsp90α, where it was shown to have a dissociation constant of 15 nM. Furthermore, the Hsp90α-NT column was applied for preliminary screening of natural Hsp90α inhibitors by zonal chromatography studies.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Whitesell L, Lidquist SL. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer. 2005;5:761–772. - PubMed
-
- Workman P. Altered states: selectively drugging the Hsp90 cancer chaperone. Trends Mol. Med. 2004;10:47–51. - PubMed
-
- Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem. Sci. 2006;31:164–172. - PubMed
-
- Kasibhatla SR, Hong K, Biamonte MA, Busch DJ, Karjian PL, Sensintaffar JL, Kamal A, Lough RE, Brekken J, Lundgren K, Grecko R, Timony GA, Mansfield RR, Fritz LC, Ulm E, Burrows FJ, Boehm MF. Rationally designed high-affinity 2-amino-6-halopurine heat shock protein 90 inhibitors that exhibit potent antitumor activity. J. Med. Chem. 2007;50:2767–2778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

