First-generation neuronal precursors in the crayfish brain are not self-renewing
- PMID: 23219763
- PMCID: PMC3619028
- DOI: 10.1016/j.ijdevneu.2012.11.010
First-generation neuronal precursors in the crayfish brain are not self-renewing
Abstract
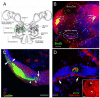
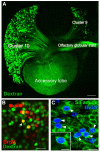
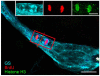
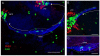
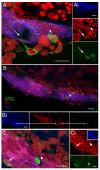
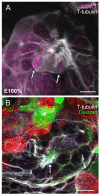
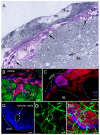
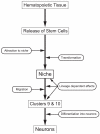
Adult-born neurons in crayfish (Procambarus clarkii) are the progeny of 1st-generation precursor cells (functionally analogous to neuronal stem cells in vertebrates) that are located in a neurogenic niche on the ventral surface of the brain. The daughters of these precursor cells migrate along the processes of bipolar niche cells to proliferation zones in the cell clusters where the somata of the olfactory interneurons reside. Here they divide again, producing offspring that differentiate into olfactory local and projection neurons. The features of this neuronal assembly line, and the fact that it continues to function when the brain is isolated and perfused or maintained in organotypic culture, provide opportunities unavailable in other organisms to explore the sequence of cellular and molecular events leading to the production of new neurons in adult brains. Further, we have determined that the 1st-generation precursor cells are not a self-renewing population, and that the niche is, nevertheless, not depleted as the animals grow and age. We conclude, therefore, that the niche is not a closed system and that there must be an extrinsic source of neuronal stem cells. Based on in vitro studies demonstrating that cells extracted from the hemolymph are attracted to the niche, as well as the intimate relationship between the niche and vasculature, we hypothesize that the hematopoietic system is a likely source of these cells.
Keywords: 5-HT; 5-bromo-2′-deoxyuridine; 5-ethynyl-2′deoxyuridine; AL; APC; BrdU; CTG; CellTracker™ Green CMFDA; EdU; GS; HPT; Hematopoietic system; Hemocytes; LPS; LPZ; MMS; MPZ; MSC; Neurogenic niche; OGT; OL; Olfactory pathway; ROS; Serotonin; Stem cell; accessory lobe; anterior proliferation center; glutamine synthetase; hematopoietic tissue; lateral proliferation zone; lipopolysaccharide; medial proliferation zone; mesenchymal stem cell; methiothepin mesylate salt; olfactory globular tract; olfactory lobe; reactive oxygen species; serotonin.
Copyright © 2012 ISDN. Published by Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Primary neuronal precursors in adult crayfish brain: replenishment from a non-neuronal source.BMC Neurosci. 2011 Jun 2;12:53. doi: 10.1186/1471-2202-12-53. BMC Neurosci. 2011. PMID: 21635768 Free PMC article.
-
5-HT receptors mediate lineage-dependent effects of serotonin on adult neurogenesis in Procambarus clarkii.Neural Dev. 2011 Jan 4;6:2. doi: 10.1186/1749-8104-6-2. Neural Dev. 2011. PMID: 21205292 Free PMC article.
-
Adult neurogenesis in the crayfish brain: the hematopoietic anterior proliferation center has direct access to the brain and stem cell niche.Stem Cells Dev. 2013 Apr 1;22(7):1027-41. doi: 10.1089/scd.2012.0583. Epub 2013 Jan 18. Stem Cells Dev. 2013. PMID: 23181901
-
Adult neurogenesis in the decapod crustacean brain: a hematopoietic connection?Eur J Neurosci. 2011 Sep;34(6):870-83. doi: 10.1111/j.1460-9568.2011.07802.x. Eur J Neurosci. 2011. PMID: 21929622 Free PMC article. Review.
-
From Blood to Brain: Adult-Born Neurons in the Crayfish Brain Are the Progeny of Cells Generated by the Immune System.Front Neurosci. 2017 Dec 7;11:662. doi: 10.3389/fnins.2017.00662. eCollection 2017. Front Neurosci. 2017. PMID: 29270102 Free PMC article. Review.
Cited by
-
A Balancing Act: The Immune System Supports Neurodegeneration and Neurogenesis.Cell Mol Neurobiol. 2020 Aug;40(6):967-989. doi: 10.1007/s10571-020-00787-5. Epub 2020 Jan 24. Cell Mol Neurobiol. 2020. PMID: 31980992 Free PMC article.
-
The Invertebrate Immunocyte: A Complex and Versatile Model for Immunological, Developmental, and Environmental Research.Cells. 2024 Dec 19;13(24):2106. doi: 10.3390/cells13242106. Cells. 2024. PMID: 39768196 Free PMC article. Review.
-
Longitudinal tracking of hemocyte populations in vivo indicates lineage relationships and supports neural progenitor identity in adult neurogenesis.Neural Dev. 2024 Jun 20;19(1):7. doi: 10.1186/s13064-024-00185-3. Neural Dev. 2024. PMID: 38902780 Free PMC article.
-
Adult neurogenesis in crayfish: Identity and regulation of neural progenitors produced by the immune system.iScience. 2022 Feb 26;25(4):103993. doi: 10.1016/j.isci.2022.103993. eCollection 2022 Apr 15. iScience. 2022. PMID: 35340434 Free PMC article.
-
Birth, survival and differentiation of neurons in an adult crustacean brain.Dev Neurobiol. 2014 Jun;74(6):602-15. doi: 10.1002/dneu.22156. Epub 2013 Dec 14. Dev Neurobiol. 2014. PMID: 24339155 Free PMC article.
References
-
- Beltz BS, Sandeman DC. Regulation of life-long neurogenesis in the decapod crustacean brain. Arthropod. Struct. Dev. 2003;32:39–60. - PubMed
-
- Blondheim NR, Levy YS, Ben-zur T, Burshtein A, Cherlow T, Kan I, Barzilai R, Bahat-Stromza M, Barhum Y, Bulvik S, Melamed E, Offen D. Human mesenchymal stem cells express neural genes, suggesting a neural predisposition. Stem Cells Dev. 2006;15:141. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

