Stomatin interacts with GLUT1/SLC2A1, band 3/SLC4A1, and aquaporin-1 in human erythrocyte membrane domains
- PMID: 23219802
- PMCID: PMC3790964
- DOI: 10.1016/j.bbamem.2012.11.030
Stomatin interacts with GLUT1/SLC2A1, band 3/SLC4A1, and aquaporin-1 in human erythrocyte membrane domains
Abstract
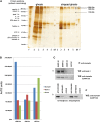
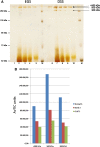
The widely expressed, homo-oligomeric, lipid raft-associated, monotopic integral membrane protein stomatin and its homologues are known to interact with and modulate various ion channels and transporters. Stomatin is a major protein of the human erythrocyte membrane, where it associates with and modifies the glucose transporter GLUT1; however, previous attempts to purify hetero-oligomeric stomatin complexes for biochemical analysis have failed. Because lateral interactions of membrane proteins may be short-lived and unstable, we have used in situ chemical cross-linking of erythrocyte membranes to fix the stomatin complexes for subsequent purification by immunoaffinity chromatography. To further enrich stomatin, we prepared detergent-resistant membranes either before or after cross-linking. Mass spectrometry of the isolated, high molecular, cross-linked stomatin complexes revealed the major interaction partners as glucose transporter-1 (GLUT1), anion exchanger (band 3), and water channel (aquaporin-1). Moreover, ferroportin-1 (SLC40A1), urea transporter-1 (SLC14A1), nucleoside transporter (SLC29A1), the calcium-pump (Ca-ATPase-4), CD47, and flotillins were identified as stomatin-interacting proteins. These findings are in line with the hypothesis that stomatin plays a role as membrane-bound scaffolding protein modulating transport proteins.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures





References
-
- Hiebl-Dirschmied C.M., Adolf G.R., Prohaska R. Isolation and partial characterization of the human erythrocyte band 7 integral membrane protein. Biochim. Biophys. Acta. 1991;1065:195–202. - PubMed
-
- Hiebl-Dirschmied C.M., Entler B., Glotzmann C., Maurer-Fogy I., Stratowa C., Prohaska R. Cloning and nucleotide sequence of cDNA encoding human erythrocyte band 7 integral membrane protein. Biochim. Biophys. Acta. 1991;1090:123–124. - PubMed
-
- Stewart G.W., Hepworth-Jones B.E., Keen J.N., Dash B.C., Argent A.C., Casimir C.M. Isolation of cDNA coding for an ubiquitous membrane protein deficient in high Na+, low K + stomatocytic erythrocytes. Blood. 1992;79:1593–1601. - PubMed
-
- Salzer U., Mairhofer M., Prohaska R. Stomatin: a new paradigm of membrane organization emerges. Dyn. Cell Biol. 2007;1:20–33.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

