Painful nerve injury decreases sarco-endoplasmic reticulum Ca²⁺-ATPase activity in axotomized sensory neurons
- PMID: 23219911
- PMCID: PMC3715030
- DOI: 10.1016/j.neuroscience.2012.11.055
Painful nerve injury decreases sarco-endoplasmic reticulum Ca²⁺-ATPase activity in axotomized sensory neurons
Abstract
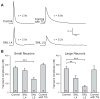
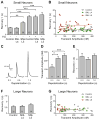
The sarco-endoplasmic reticulum Ca(2+)-ATPase (SERCA) is a critical pathway by which sensory neurons sequester cytosolic Ca(2+) and thereby maintain intracellular Ca(2+) homeostasis. We have previously demonstrated decreased intraluminal endoplasmic reticulum Ca(2+) concentration in traumatized sensory neurons. Here we examine SERCA function in dissociated sensory neurons using Fura-2 fluorometry. Blocking SERCA with thapsigargin (1 μM) increased resting [Ca(2+)](c) and prolonged recovery (τ) from transients induced by neuronal activation (elevated bath K(+)), demonstrating SERCA contributes to control of resting [Ca(2+)](c) and recovery from transient [Ca(2+)](c) elevation. To evaluate SERCA in isolation, plasma membrane Ca(2+) ATPase was blocked with pH 8.8 bath solution and mitochondrial buffering was avoided by keeping transients small (≤ 400 nM). Neurons axotomized by spinal nerve ligation (SNL) showed a slowed rate of transient recovery compared to control neurons, representing diminished SERCA function, whereas neighboring non-axotomized neurons from SNL animals were unaffected. Injury did not affect SERCA function in large neurons. Repeated depolarization prolonged transient recovery, showing that neuronal activation inhibits SERCA function. These findings suggest that injury-induced loss of SERCA function in small sensory neurons may contribute to the generation of pain following peripheral nerve injury.
Copyright © 2012 IBRO. All rights reserved.
Figures




Similar articles
-
Axotomy depletes intracellular calcium stores in primary sensory neurons.Anesthesiology. 2009 Aug;111(2):381-92. doi: 10.1097/ALN.0b013e3181ae6212. Anesthesiology. 2009. PMID: 19602958 Free PMC article.
-
Increased thrombospondin-4 after nerve injury mediates disruption of intracellular calcium signaling in primary sensory neurons.Neuropharmacology. 2017 May 1;117:292-304. doi: 10.1016/j.neuropharm.2017.02.019. Epub 2017 Feb 22. Neuropharmacology. 2017. PMID: 28232180 Free PMC article.
-
Depletion of calcium stores in injured sensory neurons: anatomic and functional correlates.Anesthesiology. 2009 Aug;111(2):393-405. doi: 10.1097/ALN.0b013e3181ae63b0. Anesthesiology. 2009. PMID: 19602957 Free PMC article.
-
Role of sarco/endoplasmic reticulum calcium content and calcium ATPase activity in the control of cell growth and proliferation.Pflugers Arch. 2009 Jan;457(3):673-85. doi: 10.1007/s00424-007-0428-7. Epub 2008 Jan 11. Pflugers Arch. 2009. PMID: 18188588 Review.
-
Role of decreased sensory neuron membrane calcium currents in the genesis of neuropathic pain.Croat Med J. 2007 Feb;48(1):9-21. Croat Med J. 2007. PMID: 17309135 Free PMC article. Review.
Cited by
-
Analgesic dorsal root ganglionic field stimulation blocks conduction of afferent impulse trains selectively in nociceptive sensory afferents.Pain. 2020 Dec;161(12):2872-2886. doi: 10.1097/j.pain.0000000000001982. Pain. 2020. PMID: 32658148 Free PMC article.
-
Calcium homeostasis in parvalbumin DRG neurons is altered after sciatic nerve crush and sciatic nerve transection injuries.J Neurophysiol. 2021 Dec 1;126(6):1948-1958. doi: 10.1152/jn.00707.2020. Epub 2021 Nov 10. J Neurophysiol. 2021. PMID: 34758279 Free PMC article.
-
Pathological pain processing in mouse models of multiple sclerosis and spinal cord injury: contribution of plasma membrane calcium ATPase 2 (PMCA2).J Neuroinflammation. 2019 Nov 8;16(1):207. doi: 10.1186/s12974-019-1585-2. J Neuroinflammation. 2019. PMID: 31703709 Free PMC article.
-
Sensory neuron dysfunction in orthotopic mouse models of colon cancer.J Neuroinflammation. 2022 Aug 12;19(1):204. doi: 10.1186/s12974-022-02566-z. J Neuroinflammation. 2022. PMID: 35962398 Free PMC article.
-
CaMKII Controls Whether Touch Is Painful.J Neurosci. 2015 Oct 21;35(42):14086-102. doi: 10.1523/JNEUROSCI.1969-15.2015. J Neurosci. 2015. PMID: 26490852 Free PMC article.
References
-
- Albrecht MA, Colegrove SL, Hongpaisan J, Pivovarova NB, Andrews SB, Friel DD. Multiple modes of calcium-induced calcium release in sympathetic neurons: I. Attenuation of endoplasmic reticulum Ca2+ accumulation at low [Ca2+](i) during weak depolarization. J Gen Physiol. 2001;118:83–100. - PMC - PubMed
-
- Avery RB, Johnston D. Ca2+ channel antagonist U-92032 inhibits both T-type Ca2+ channels and Na+ channels in hippocampal CA1 pyramidal neurons. J Neurophysiol. 1997;77:1023–1028. - PubMed
-
- Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341–1378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

