Distinct signaling pathways regulate TLR2 co-stimulatory function in human T cells
- PMID: 23219913
- PMCID: PMC3883577
- DOI: 10.1016/j.cellsig.2012.11.026
Distinct signaling pathways regulate TLR2 co-stimulatory function in human T cells
Abstract
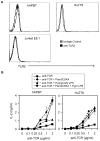
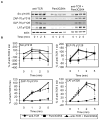
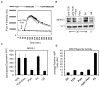
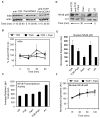
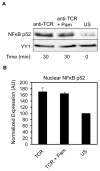
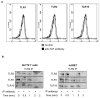
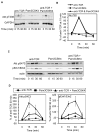
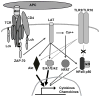
Toll-like receptor 2 (TLR2) serves as a co-stimulatory receptor for human T cells by enhancing T cell receptor (TCR)-induced cytokine production and proliferation. However, it is unknown where signals from the TCR and TLR2 converge to enhance T cell activation. To address this gap, we examined changes in TCR-induced signaling following concurrent TLR2 activation in human T cells. Both proximal TCR-mediated signaling and early NFκB activation were not enhanced by TCR and TLR2 co-activation, potentially due to the association of TLR2 with TLR10. Instead, TLR2 co-induction did augment Akt and Erk1/Erk2 activation in human T cells. These findings demonstrate that TLR2 activates distinct signaling pathways in human T cells and suggest that alterations in expression of TLR2 co-receptors may contribute to aberrant T cell responses.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

