3-(1H-indol-3-yl)-2-[3-(4-nitrophenyl)ureido]propanamide enantiomers with human formyl-peptide receptor agonist activity: molecular modeling of chiral recognition by FPR2
- PMID: 23219934
- PMCID: PMC3553303
- DOI: 10.1016/j.bcp.2012.11.015
3-(1H-indol-3-yl)-2-[3-(4-nitrophenyl)ureido]propanamide enantiomers with human formyl-peptide receptor agonist activity: molecular modeling of chiral recognition by FPR2
Abstract
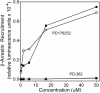
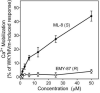
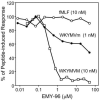
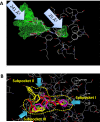
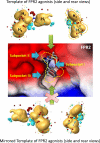
N-formyl peptide receptors (FPRs) are G protein-coupled receptors (GPCRs) that play critical roles in inflammatory reactions, and FPR-specific interactions can possibly be used to facilitate the resolution of pathological inflammatory reactions. Recent studies indicated that FPRs have stereo-selective preference for chiral ligands. Here, we investigated the structure-activity relationship of 24 chiral ureidopropanamides, including previously reported compounds PD168368/PD176252 and their close analogs, and used molecular modeling to define chiral recognition by FPR2. Unlike previously reported 6-methyl-2,4-disubstituted pyridazin-3(2H)-ones, whose R-forms preferentially activated FPR1/FPR2, we found that four S-enantiomers in the seven ureidopropanamide pairs tested preferentially activated intracellular Ca(2+) flux in FPR2-transfected cells, while the R-counterpart was more active in two enantiomer pairs. Thus, active enantiomers of FPR2 agonists can be in either R- or S-configurations, depending on the molecular scaffold and specific substituents at the chiral center. Using molecular modeling approaches, including field point methodology, homology modeling, and docking studies, we propose a model that can explain stereoselective activity of chiral FPR2 agonists. Importantly, our docking studies of FPR2 chiral agonists correlated well with the FPR2 pharmacophore model derived previously. We conclude that the ability of FPR2 to discriminate between the enantiomers is the consequence of the arrangement of the three asymmetric hydrophobic subpockets at the main orthosteric FPR2 binding site with specific orientation of charged regions in the subpockets.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Gao JL, Murphy PM. Species and subtype variants of the N-formyl peptide chemotactic receptor reveal multiple important functional domains. J Biol Chem. 1993;268:25395–401. - PubMed
-
- Migeotte I, Communi D, Parmentier M. Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006;17:501–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

