Gastrointestinal hormones stimulate growth of Foregut Neuroendocrine Tumors by transactivating the EGF receptor
- PMID: 23220008
- PMCID: PMC3556220
- DOI: 10.1016/j.bbamcr.2012.11.021
Gastrointestinal hormones stimulate growth of Foregut Neuroendocrine Tumors by transactivating the EGF receptor
Abstract
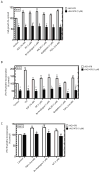
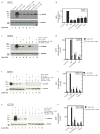
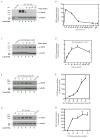
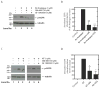
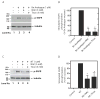
Foregut neuroendocrine tumors [NETs] usually pursuit a benign course, but some show aggressive behavior. The treatment of patients with advanced NETs is marginally effective and new approaches are needed. In other tumors, transactivation of the EGF receptor (EGFR) by growth factors, gastrointestinal (GI) hormones and lipids can stimulate growth, which has led to new treatments. Recent studies show a direct correlation between NET malignancy and EGFR expression, EGFR inhibition decreases basal NET growth and an autocrine growth effect exerted by GI hormones, for some NETs. To determine if GI hormones can stimulate NET growth by inducing transactivation of EGFR, we examined the ability of EGF, TGFα and various GI hormones to stimulate growth of the human foregut carcinoid,BON, the somatostatinoma QGP-1 and the rat islet tumor,Rin-14B-cell lines. The EGFR tyrosine-kinase inhibitor, AG1478 strongly inhibited EGF and the GI hormones stimulated cell growth, both in BON and QGP-1 cells. In all the three neuroendocrine cell lines studied, we found EGF, TGFα and the other growth-stimulating GI hormones increased Tyr(1068) EGFR phosphorylation. In BON cells, both the GI hormones neurotensin and a bombesin analogue caused a time- and dose-dependent increase in EGFR phosphorylation, which was strongly inhibited by AG1478. Moreover, we found this stimulated phosphorylation was dependent on Src kinases, PKCs, matrix metalloproteinase activation and the generation of reactive oxygen species. These results raise the possibility that disruption of this signaling cascade by either EGFR inhibition alone or combined with receptor antagonists may be a novel therapeutic approach for treatment of foregut NETs/PETs.
Published by Elsevier B.V.
Figures
References
-
- Jensen RT, Delle Fave G. Promising advances in the treatment of malignant pancreatic endocrine tumors. N Engl J Med. 2011;364:564–565. - PubMed
-
- Schimmack S, Svejda B, Lawrence B, Kidd M, Modlin IM. The diversity and commonalities of gastroenteropancreatic neuroendocrine tumors. Langenbecks Arch Surg. 2011;396:273–298. - PubMed
-
- Capurso G, Fazio N, Festa S, Panzuto F, de Braud F, Delle Fave G. Molecular target therapy for gastroenteropancreatic endocrine tumours: biological rationale and clinical perspectives. Crit Rev Oncol Hematol. 2009;72:110–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

