CaV1.2 sparklets in heart and vascular smooth muscle
- PMID: 23220157
- PMCID: PMC3678956
- DOI: 10.1016/j.yjmcc.2012.11.018
CaV1.2 sparklets in heart and vascular smooth muscle
Abstract
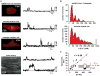
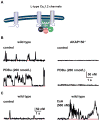
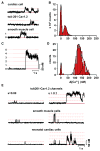
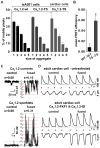
CaV1.2 sparklets are local elevations in intracellular Ca(2+) ([Ca(2+)]i) resulting from the opening of a single or small cluster of voltage-gated, dihydropyridine-sensitive CaV1.2 channels. Activation of CaV1.2 sparklets is an early event in the signaling cascade that couples membrane depolarization to contraction (i.e., excitation-contraction coupling) in cardiac and arterial smooth muscle. Here, we review recent work on the molecular and biophysical mechanisms that regulate CaV1.2 sparklet activity in these cells. CaV1.2 sparklet activity is tightly regulated by a cohort of protein kinases and phosphatases that are targeted to specific regions of the sarcolemma by the anchoring protein AKAP150. We discuss a model for the local control of Ca(2+) influx via CaV1.2 channels in which a signaling complex formed by AKAP79/150, protein kinase C, protein kinase A, and calcineurin regulates the activity of individual CaV1.2 channels and also facilitates the coordinated activation of small clusters of these channels. This results in amplification of Ca(2+) influx, which strengthens excitation-contraction coupling in cardiac and vascular smooth muscle.
Copyright © 2012. Published by Elsevier Ltd.
Figures

References
-
- Guatimosim S, Dilly K, Santana LF, Saleet Jafri M, Sobie E, Lederer W. Local Ca2+ Signaling and EC Coupling in Heart: Ca2+ Sparks and the Regulation of the [Ca2+]i Transient. Journal of molecular and cellular cardiology. 2002;34:941. - PubMed
-
- Gollasch M, Lohn M, Furstenau M, Nelson MT, Luft FC, Haller H. Ca2+ channels, ‘quantized’ Ca2+ release, and differentiation of myocytes in the cardiovascular system. J Hypertens. 2000;18:989–98. - PubMed
-
- Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

