Metabolic abnormalities and body composition of HIV-infected children on Lopinavir or Nevirapine-based antiretroviral therapy
- PMID: 23220209
- PMCID: PMC4533934
- DOI: 10.1136/archdischild-2012-302633
Metabolic abnormalities and body composition of HIV-infected children on Lopinavir or Nevirapine-based antiretroviral therapy
Abstract
Background: Few studies have assessed metabolic and body composition alterations in perinatally HIV-infected African children on antiretroviral therapy (ART). We compared metabolic profiles and regional fat of children on ritonavir-boosted lopinavir (lopinavir/ritonavir), lamivudine and stavudine to those switched to nevirapine, lamivudine and stavudine.
Methods: This study evaluated metabolic and body composition outcomes in 156 HIV-infected children completing a randomised trial that assessed the continued use of lopinavir/ritonavir-based ART or switch to nevirapine-based ART in Johannesburg, South Africa (2005-2010). Fasting total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides total and regional body fat (BF) were measured. A clinical assessment for lipodystrophy (LD) was conducted.
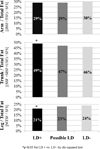
Results: 156 children (mean age 5.1±0.8 years, mean duration of treatment 4.2±0.7 years, mean time since randomisation 3.4±0.7 years) were enrolled. 85 were randomised to the lopinavir/ritonavir group and 71 to the nevirapine group. The lopinavir/ritonavir group had lower mean HDL (1.3±0.4 vs 1.5±0.4 mmol/l, p<0.001) and higher mean TC (4.4±1.0 vs 4.1±0.8 mmol/l, p=0.097), LDL (2.6±0.9 vs 2.3±0.7 mmol/l, p=0.018) and triglycerides (1.1±0.4 vs 0.8±0.3 mmol/l, p<0.001). The lopinavir/ritonavir group had more total BF by mean skinfold sum (43±11.1 vs 39±10.1 mm, p=0.031) and BF% by bioelectrical impedance analysis (17.0±7.0 vs 14.1±8.0%, p=0.022). Thirteen (8.4%) met criteria for LD.
Conclusions: Unfavourable alterations in lipid profile and triglycerides, and differences in fat are detectable in young HIV-infected South African children receiving lopinavir/ritonavir-based regimens versus those switched to nevirapine-based regimens. Interventions to mitigate these alterations are warranted to reduce long-term cardiovascular disease risk.
Figures

Comment in
-
Complications of long-term antiretroviral therapy in HIV-infected children.Arch Dis Child. 2013 Apr;98(4):245-6. doi: 10.1136/archdischild-2012-303215. Epub 2013 Feb 14. Arch Dis Child. 2013. PMID: 23413313 No abstract available.
Similar articles
-
Sex differences in responses to antiretroviral treatment in South African HIV-infected children on ritonavir-boosted lopinavir- and nevirapine-based treatment.BMC Pediatr. 2014 Feb 12;14:39. doi: 10.1186/1471-2431-14-39. BMC Pediatr. 2014. PMID: 24521425 Free PMC article. Clinical Trial.
-
Zidovudine/lamivudine for HIV-1 infection contributes to limb fat loss.PLoS One. 2009 May 21;4(5):e5647. doi: 10.1371/journal.pone.0005647. PLoS One. 2009. PMID: 19479079 Free PMC article. Clinical Trial.
-
Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial.JAMA. 2010 Sep 8;304(10):1082-90. doi: 10.1001/jama.2010.1278. JAMA. 2010. PMID: 20823434 Free PMC article. Clinical Trial.
-
Recent advances in pharmacovigilance of antiretroviral therapy in HIV-infected and exposed children.Curr Opin HIV AIDS. 2012 Jul;7(4):305-16. doi: 10.1097/COH.0b013e328354da1d. Curr Opin HIV AIDS. 2012. PMID: 22678488 Review.
-
Protease inhibitor-based antiretroviral therapy in pregnancy: effects on hormones, placenta, and decidua.Lancet HIV. 2022 Feb;9(2):e120-e129. doi: 10.1016/S2352-3018(21)00249-6. Epub 2021 Dec 2. Lancet HIV. 2022. PMID: 34863352 Review.
Cited by
-
Decreased Vigorous Physical Activity in School-Aged Children with Human Immunodeficiency Virus in Johannesburg, South Africa.J Pediatr. 2016 May;172:103-9. doi: 10.1016/j.jpeds.2016.01.034. Epub 2016 Feb 26. J Pediatr. 2016. PMID: 26922104 Free PMC article.
-
Paediatric European Network for Treatment of AIDS (PENTA) guidelines for treatment of paediatric HIV-1 infection 2015: optimizing health in preparation for adult life.HIV Med. 2018 Jan;19(1):e1-e42. doi: 10.1111/hiv.12217. Epub 2015 Feb 3. HIV Med. 2018. PMID: 25649230 Free PMC article.
-
The changing epidemiology of the global paediatric HIV epidemic: keeping track of perinatally HIV-infected adolescents.J Int AIDS Soc. 2013 Jun 18;16(1):18555. doi: 10.7448/IAS.16.1.18555. J Int AIDS Soc. 2013. PMID: 23782474 Free PMC article. Review.
-
Antiretroviral Therapy-Induced Dysregulation of Gene Expression and Lipid Metabolism in HIV+ Patients: Beneficial Role of Antioxidant Phytochemicals.Int J Mol Sci. 2022 May 17;23(10):5592. doi: 10.3390/ijms23105592. Int J Mol Sci. 2022. PMID: 35628408 Free PMC article. Review.
-
Induction with lopinavir-based treatment followed by switch to nevirapine-based regimen versus non-nucleoside reverse transcriptase inhibitors-based treatment for first line antiretroviral therapy in HIV infected children three years and older.PLoS One. 2014 Sep 18;9(9):e108063. doi: 10.1371/journal.pone.0108063. eCollection 2014. PLoS One. 2014. PMID: 25232730 Free PMC article.
References
-
- Carter RJ, Wiener J, Abrams EJ, et al. Dyslipidemia among perinatally HIV-infected children enrolled in the PACTS-HOPE cohort: 1999–2004: a longitudinal analysis. J Acquir Immune Defic Syndr. 2006;41(4):453. - PubMed
-
- Sztam KA, Jiang H, Jurgrau A, Deckelbaum RJ, Foca MD. Early Increases in Concentrations of Total, LDL, and HDL Cholesterol in HIV-infected Children Following New Exposure to Antiretroviral Therapy. J Pediatr Gastroenterol Nutr. 2011;52(4):495. - PubMed
-
- Jaquet D, Lévine M, Ortega-Rodriguez E, et al. Clinical and metabolic presentation of the lipodystrophic syndrome in HIV-infected children. AIDS. 2000;14(14):2123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
