Resident mesenchymal cells and fibrosis
- PMID: 23220259
- PMCID: PMC3672235
- DOI: 10.1016/j.bbadis.2012.11.015
Resident mesenchymal cells and fibrosis
Abstract
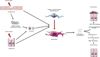
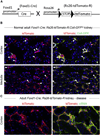
Fibrosis is a major clinical problem associated with as many as 45% of all natural deaths in developed nations. It can affect all organs and accumulating evidence indicates that fibrogenesis is not merely a bystander product of injury, but is a central pathological problem directly contributing to loss of organ function. In the majority of clinical cases, fibrogenesis is strongly associated with the recruitment of leukocytes, even in the absence of infection. Although chronic infections are a significant cause of fibrogenesis, in most cases fibrotic disease occurs in the context of sterile injury, such as microvascular disease, toxic epithelial injury or diabetes mellitus. Fibrogenesis is a direct consequence of the activation of extensive, and previously poorly appreciated, populations of mesenchymal cells in our organs which are either wrapped around capillaries and known as 'pericytes', or embedded in interstitial spaces between cell structures and known as resident 'fibroblasts'. Recent fate-mapping and complementary studies in several organs indicate that these cells are the precursors of the scar-forming myofibroblasts that appear in our organs in response to injury. Here we will review the literature supporting a central role for these cells in fibrogenesis, and highlight some of the critical cell to cell interactions that are necessary for the initiation and continuation of the fibrogenic process. This article is part of a Special Issue entitled: Fibrosis: Translation of basic research to human disease.
Copyright © 2012 Elsevier B.V. All rights reserved.
Conflict of interest statement
JSD is on the Scientific Advisory Board of Promedior Inc., and Regulus Therapeutics. He is the founder of Muregen LLC. He has consulted with Takeda, Boehringer Ingelheim, Bristol Mayer Squibb, GlaxoSmithKline, Biogen Idec and Gilead Pharmaceutical Companies.
Figures


References
-
- Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. - PubMed
-
- Selman M, Rojas M, Mora A, Pardo A. Aging and interstitial lung diseases: unraveling an old forgotten player in the pathogenesis of lung fibrosis. Semin. Respir. Crit. Care Med. 2010;31:607–617. - PubMed
-
- Monroe DM, Hoffman M. The clotting system – a major player in wound healing. Haemophilia. 2012;18:11–16. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

