Direct assessment of articular cartilage and underlying subchondral bone reveals a progressive gene expression change in human osteoarthritic knees
- PMID: 23220557
- PMCID: PMC3593157
- DOI: 10.1016/j.joca.2012.11.016
Direct assessment of articular cartilage and underlying subchondral bone reveals a progressive gene expression change in human osteoarthritic knees
Abstract
Objective: To evaluate the interaction of articular cartilage (AC) and subchondral bone (SB) through analysis of osteoarthritis (OA)-related genes of site-matched tissue.
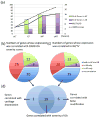
Design: We developed a novel method for isolating site-matched overlying AC and underlying SB from three and four regions of interest respectively from the human knee tibial plateau (n = 50). For each site, the severity of cartilage changes of OA were assessed histologically, and the severity of bone abnormalities were assessed by microcomputed tomography. An RNA isolation procedure was optimized that yielded high quality RNA from site-matched AC and SB tibial regions. Quantitative polymerase chain reaction (Q-PCR) analysis was performed to evaluate gene expression of 61 OA-associated genes for correlation with cartilage integrity and bone structure parameters.
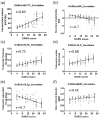
Results: A total of 27 (44%) genes were coordinately up- or down-regulated in both tissues. The expression levels of 19 genes were statistically significantly correlated with the severity of AC degeneration and changes of SB structure; these included: ADAMTS1, ASPN, BMP6, BMPER, CCL2, CCL8, COL5A1, COL6A3, COL7A1, COL16A1, FRZB, GDF10, MMP3, OGN, OMD, POSTN, PTGES, TNFSF11 and WNT1.
Conclusions: These results provide a strategy for identifying targets whose modification may have the potential to ameliorate pathological alterations and progression of disease in both AC and SB simultaneously. In addition, this is the first study, to our knowledge, to overcome the major difficulties related to isolation of high quality RNA from site-matched joint tissues. We expect this method to facilitate advances in our understanding of the coordinated molecular responses of the whole joint organ.
Copyright © 2012 Osteoarthritis Research Society International. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Grynpas MD, Alpert B, Katz I, Lieberman I, Pritzker KP. Subchondral bone in osteoarthritis. Calcif Tissue Int. 1991;49:20–26. - PubMed
-
- Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–237. - PubMed
-
- Herrero-Beaumont G, Roman-Blas JA, Largo R, Berenbaum F, Castaneda S. Bone mineral density and joint cartilage: four clinical settings of a complex relationship in osteoarthritis. Ann Rheum Dis. 2011;70:1523–1525. - PubMed
-
- Ding C, Cicuttini F, Jones G. Tibial subchondral bone size and knee cartilage defects: relevance to knee osteoarthritis. Osteoarthritis Cartilage. 2007;15:479–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

