Overexpression of methyl-CpG binding protein 2 impairs T(H)1 responses
- PMID: 23220634
- PMCID: PMC3628825
- DOI: 10.1126/scitranslmed.3004430
Overexpression of methyl-CpG binding protein 2 impairs T(H)1 responses
Abstract
The DNA binding protein methyl-CpG binding protein 2 (MeCP2) critically influences neuronal and brain function by modulating gene expression, and children with overexpression of the MECP2 gene exhibit postnatal neurological syndromes. We demonstrate that some children with MECP2 duplication also display variable immunological abnormalities that include reductions in memory T and B cells and natural killer cells and immunoglobulin assay responses. Moreover, whereas mice with MeCP2 overexpression were unable to control infection with the intra-macrophage parasite Leishmania major and secrete interferon-γ (IFN-γ) from involved lymph nodes, they were able to control airway fungal infection by Aspergillus niger and mount protective T helper cell type 2 (T(H)2)-dependent allergic responses. Relative to normal T cells, T(H) cells from children and mice with MECP2 duplication displayed similar impairments in IFN-γ secretion and T(H)1 responses that were due to both MeCP2-dependent suppression of IFN-γ transcription and sequestration of the IFN-γ locus as assessed by chromatin immunoprecipitation assay. Thus, overexpressed MeCP2 aberrantly suppresses IFN-γ secretion from T(H) cells, potentially leading to a partially immunodeficient state. Our findings establish a rational basis for identifying, treating, and preventing infectious complications potentially affecting children with MECP2 duplication.
Figures

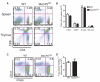

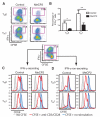
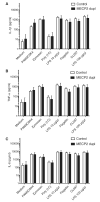

Comment in
-
The Goldilocks effect.Sci Transl Med. 2012 Dec 5;4(163):163fs42. doi: 10.1126/scitranslmed.3005280. Sci Transl Med. 2012. PMID: 23220631
-
Overexpression of methyl-CpG-binding protein 2 and autoimmunity: evidence from MECP2 duplication syndrome, lupus, MECP2 transgenic and Mecp2 deficient mice.Lupus. 2013 Aug;22(9):870-2. doi: 10.1177/0961203313497119. Epub 2013 Jul 16. Lupus. 2013. PMID: 23861028 Free PMC article.
References
-
- Guy J, Cheval H, Selfridge J, Bird A. The role of MeCP2 in the brain. Annu. Rev. Cell Dev. Biol. 2011;27:631–652. - PubMed
-
- Lubs H, Abidi F, Bier JA, Abuelo D, Ouzts L, Voeller K, Fennell E, Stevenson RE, Schwartz CE, Arena F. XLMR syndrome characterized by multiple respiratory infections, hypertelorism, severe CNS deterioration and early death localizes to distal Xq28. Am. J. Med. Genet. 1999;85:243–248. - PubMed
-
- Van Esch H, Bauters M, Ignatius J, Jansen M, Raynaud M, Hollanders K, Lugtenberg D, Bienvenu T, Jensen LR, Gecz J, Moraine C, Marynen P, Fryns JP, Froyen G. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am. J. Hum. Genet. 2005;77:442–453. - PMC - PubMed
-
- Carvalho CM, Zhang F, Liu P, Patel A, Sahoo T, Bacino CA, Shaw C, Peacock S, Pursley A, Tavyev YJ, Ramocki MB, Nawara M, Obersztyn E, Vianna-Morgante AM, Stankiewicz P, Zoghbi HY, Cheung SW, Lupski JR. Complex rearrangements in patients with duplications of MECP2 can occur by fork stalling and template switching. Hum. Mol. Genet. 2009;18:2188–2203. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI057696/AI/NIAID NIH HHS/United States
- AI070973/AI/NIAID NIH HHS/United States
- NS057819/NS/NINDS NIH HHS/United States
- R01 AI057696/AI/NIAID NIH HHS/United States
- P30 HD024064/HD/NICHD NIH HHS/United States
- T32 NS043124/NS/NINDS NIH HHS/United States
- HL75243/HL/NHLBI NIH HHS/United States
- U19 AI070973/AI/NIAID NIH HHS/United States
- K02 HL075243/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- HD024064/HD/NICHD NIH HHS/United States
- R01 HD062553/HD/NICHD NIH HHS/United States
- K08 NS062711/NS/NINDS NIH HHS/United States
- R01 NS057819/NS/NINDS NIH HHS/United States
- NS062711/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

