Circulating histones are mediators of trauma-associated lung injury
- PMID: 23220920
- PMCID: PMC3570656
- DOI: 10.1164/rccm.201206-1037OC
Circulating histones are mediators of trauma-associated lung injury
Abstract
Rationale: Acute lung injury is a common complication after severe trauma, which predisposes patients to multiple organ failure. This syndrome largely accounts for the late mortality that arises and despite many theories, the pathological mechanism is not fully understood. Discovery of histone-induced toxicity in mice presents a new dimension for elucidating the underlying pathophysiology.
Objectives: To investigate the pathological roles of circulating histones in trauma-induced lung injury.
Methods: Circulating histone levels in patients with severe trauma were determined and correlated with respiratory failure and Sequential Organ Failure Assessment (SOFA) scores. Their cause-effect relationship was studied using cells and mouse models.
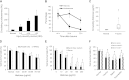
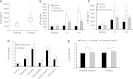
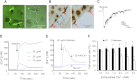
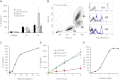
Measurements and main results: In a cohort of 52 patients with severe nonthoracic blunt trauma, circulating histones surged immediately after trauma to levels that were toxic to cultured endothelial cells. The high levels were significantly associated with the incidence of acute lung injury and SOFA scores, as well as markers of endothelial damage and coagulation activation. In in vitro systems, histones damaged endothelial cells, stimulated cytokine release, and induced neutrophil extracellular trap formation and myeloperoxidase release. Cellular toxicity resulted from their direct membrane interaction and resultant calcium influx. In mouse models, cytokines and markers for endothelial damage and coagulation activation significantly increased immediately after trauma or histone infusion. Pathological examinations showed that lungs were the predominantly affected organ with edema, hemorrhage, microvascular thrombosis, and neutrophil congestion. An anti-histone antibody could reduce these changes and protect mice from histone-induced lethality.
Conclusions: This study elucidates a new mechanism for acute lung injury after severe trauma and proposes that circulating histones are viable therapeutic targets for improving survival outcomes in patients.
Figures


Comment in
-
Circulating histones: a novel target in acute respiratory distress syndrome?Am J Respir Crit Care Med. 2013 Jan 15;187(2):118-20. doi: 10.1164/rccm.201211-2025ED. Am J Respir Crit Care Med. 2013. PMID: 23322791 No abstract available.
References
-
- Gomberg BF, Gruen GS, Smith WR, Spott M. Outcomes in acute orthopaedic trauma: a review of 130,506 patients by age. Injury 1999;30:431–437 - PubMed
-
- Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. Injury 2009;40:912–918 - PubMed
-
- Tsukamoto T, Chanthaphavong RS, Pape HC. Current theories on the pathophysiology of multiple organ failure after trauma. Injury 2010;41:21–26 - PubMed
-
- Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. role of the lung in postinjury multiple organ failure. Surgery 2005;138:749–757; discussion 757–748 - PubMed
-
- Martin AM, Jr, Soloway HB, Simmons RL. Pathologic anatomy of the lungs following shock and trauma. J Trauma 1968;8:687–699 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

