Host responses to persistent Mycobacterium avium subspecies paratuberculosis infection in surgically isolated bovine ileal segments
- PMID: 23221000
- PMCID: PMC3571287
- DOI: 10.1128/CVI.00496-12
Host responses to persistent Mycobacterium avium subspecies paratuberculosis infection in surgically isolated bovine ileal segments
Abstract
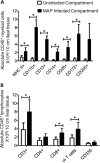
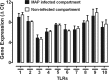
A lack of appropriate disease models has limited our understanding of the pathogenesis of persistent enteric infections with Mycobacterium avium subsp. paratuberculosis. A model was developed for the controlled delivery of a defined dose of M. avium subsp. paratuberculosis to surgically isolated ileal segments in newborn calves. The stable intestinal segments enabled the characterization of host responses to persistent M. avium subsp. paratuberculosis infections after a 9-month period, including an analysis of local mucosal immune responses relative to an adjacent uninfected intestinal compartment. M. avium subsp. paratuberculosis remained localized at the initial site of intestinal infection and was not detected by PCR in the mesenteric lymph node. M. avium subsp. paratuberculosis-specific T cell proliferative responses included both CD4 and γδ T cell receptor (γδTcR) T cell responses in the draining mesenteric lymph node. The levels of CD8(+) and γδTcR(+) T cells increased significantly (P < 0.05) in the lamina propria, and M. avium subsp. paratuberculosis-specific tumor necrosis factor alpha (TNF-α) and gamma interferon secretion by lamina propria leukocytes was also significantly (P < 0.05) increased. There was a significant (P < 0.05) accumulation of macrophages and dendritic cells (DCs) in the lamina propria, but the expression of mucosal toll-like receptors 1 through 10 was not significantly changed by M. avium subsp. paratuberculosis infection. In conclusion, surgically isolated ileal segments provided a model system for the establishment of a persistent and localized enteric M. avium subsp. paratuberculosis infection in cattle and facilitated the analysis of M. avium subsp. paratuberculosis-specific changes in mucosal leukocyte phenotype and function. The accumulation of DC subpopulations in the lamina propria suggests that further investigation of mucosal DCs may provide insight into host responses to M. avium subsp. paratuberculosis infection and improve vaccine strategies to prevent M. avium subsp. paratuberculosis infection.
Figures




Similar articles
-
Marked Differences in Mucosal Immune Responses Induced in Ileal versus Jejunal Peyer's Patches to Mycobacterium avium subsp. paratuberculosis Secreted Proteins following Targeted Enteric Infection in Young Calves.PLoS One. 2016 Jul 7;11(7):e0158747. doi: 10.1371/journal.pone.0158747. eCollection 2016. PLoS One. 2016. PMID: 27387969 Free PMC article.
-
Cytokine gene expression in peripheral blood mononuclear cells and tissues of cattle infected with Mycobacterium avium subsp. paratuberculosis: evidence for an inherent proinflammatory gene expression pattern.Infect Immun. 2004 Mar;72(3):1409-22. doi: 10.1128/IAI.72.3.1409-1422.2004. Infect Immun. 2004. PMID: 14977946 Free PMC article.
-
Bovine Immunoinhibitory Receptors Contribute to Suppression of Mycobacterium avium subsp. paratuberculosis-Specific T-Cell Responses.Infect Immun. 2015 Oct 19;84(1):77-89. doi: 10.1128/IAI.01014-15. Print 2016 Jan. Infect Immun. 2015. PMID: 26483406 Free PMC article.
-
Immunology of mycobacterial infections: with special reference to Mycobacterium avium subspecies paratuberculosis.Immunobiology. 2008;213(7):585-98. doi: 10.1016/j.imbio.2007.11.002. Epub 2008 Mar 4. Immunobiology. 2008. PMID: 18656706 Review.
-
Regulatory T cells in cattle and their potential role in bovine paratuberculosis.Comp Immunol Microbiol Infect Dis. 2012 May;35(3):233-9. doi: 10.1016/j.cimid.2012.01.004. Epub 2012 Jan 27. Comp Immunol Microbiol Infect Dis. 2012. PMID: 22285689 Review.
Cited by
-
Johne's Disease in Dairy Cattle: An Immunogenetic Perspective.Front Vet Sci. 2021 Aug 26;8:718987. doi: 10.3389/fvets.2021.718987. eCollection 2021. Front Vet Sci. 2021. PMID: 34513975 Free PMC article. Review.
-
From mouth to macrophage: mechanisms of innate immune subversion by Mycobacterium avium subsp. paratuberculosis.Vet Res. 2014 May 15;45(1):54. doi: 10.1186/1297-9716-45-54. Vet Res. 2014. PMID: 24885748 Free PMC article. Review.
-
The within host dynamics of Mycobacterium avium ssp. paratuberculosis infection in cattle: where time and place matter.Vet Res. 2015 Jun 19;46(1):61. doi: 10.1186/s13567-015-0185-0. Vet Res. 2015. PMID: 26092382 Free PMC article. Review.
-
Regional Dichotomy in Enteric Mucosal Immune Responses to a Persistent Mycobacterium avium ssp. paratuberculosis Infection.Front Immunol. 2020 May 29;11:1020. doi: 10.3389/fimmu.2020.01020. eCollection 2020. Front Immunol. 2020. PMID: 32547548 Free PMC article.
-
From Beef to Bees: High-Throughput Kinome Analysis to Understand Host Responses of Livestock Species to Infectious Diseases and Industry-Associated Stress.Front Immunol. 2020 May 15;11:765. doi: 10.3389/fimmu.2020.00765. eCollection 2020. Front Immunol. 2020. PMID: 32499776 Free PMC article. Review.
References
-
- Coussens PM. 2001. Mycobacterium paratuberculosis and the bovine immune system. Anim. Health Res. Rev. 2:141–161 - PubMed
-
- Stabel JR. 2006. Host responses to Mycobacterium avium subsp. paratuberculosis: a complex arsenal. Anim. Health Res. Rev. 7:61–70 - PubMed
-
- Ott SL, Wells SJ, Wagner BA. 1999. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev. Vet. Med. 40:179–192 - PubMed
-
- Pradhan AK, Mitchell RM, Kramer AJ, Zurakowski MJ, Fyock TL, Whitlock RH, Smith JM, Hovingh E, Van Kessel JA, Karns JS, Schukken YH. 2011. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis in a longitudinal study of three dairy herds. J. Clin. Microbiol. 49:893–901 - PMC - PubMed
-
- Pierce ES. 2009. Where are all the Mycobacterium avium subspecies paratuberculosis in patients with Crohn's disease? PLoS Pathog. 5:e1000234 doi:10.1371/journal.ppat.1000234 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

