Vertical T cell immunodominance and epitope entropy determine HIV-1 escape
- PMID: 23221345
- PMCID: PMC3533301
- DOI: 10.1172/JCI65330
Vertical T cell immunodominance and epitope entropy determine HIV-1 escape
Abstract
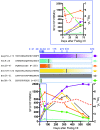
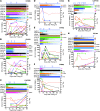
HIV-1 accumulates mutations in and around reactive epitopes to escape recognition and killing by CD8+ T cells. Measurements of HIV-1 time to escape should therefore provide information on which parameters are most important for T cell-mediated in vivo control of HIV-1. Primary HIV-1-specific T cell responses were fully mapped in 17 individuals, and the time to virus escape, which ranged from days to years, was measured for each epitope. While higher magnitude of an individual T cell response was associated with more rapid escape, the most significant T cell measure was its relative immunodominance measured in acute infection. This identified subject-level or "vertical" immunodominance as the primary determinant of in vivo CD8+ T cell pressure in HIV-1 infection. Conversely, escape was slowed significantly by lower population variability, or entropy, of the epitope targeted. Immunodominance and epitope entropy combined to explain half of all the variability in time to escape. These data explain how CD8+ T cells can exert significant and sustained HIV-1 pressure even when escape is very slow and that within an individual, the impacts of other T cell factors on HIV-1 escape should be considered in the context of immunodominance.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

