Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses
- PMID: 23221383
- PMCID: PMC3563931
- DOI: 10.1158/0008-5472.CAN-12-2731
Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses
Abstract
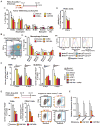
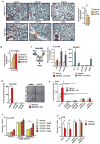
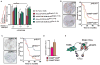
Tumor-infiltrating immune cells can promote chemoresistance and metastatic spread in aggressive tumors. Consequently, the type and quality of immune responses present in the neoplastic stroma are highly predictive of patient outcome in several cancer types. In addition to host immune responses, intrinsic tumor cell activities that mimic stem cell properties have been linked to chemoresistance, metastatic dissemination, and the induction of immune suppression. Cancer stem cells are far from a static cell population; rather, their presence seems to be controlled by highly dynamic processes that are dependent on cues from the tumor stroma. However, the impact immune responses have on tumor stem cell differentiation or expansion is not well understood. In this study, we show that targeting tumor-infiltrating macrophages (TAM) and inflammatory monocytes by inhibiting either the myeloid cell receptors colony-stimulating factor-1 receptor (CSF1R) or chemokine (C-C motif) receptor 2 (CCR2) decreases the number of tumor-initiating cells (TIC) in pancreatic tumors. Targeting CCR2 or CSF1R improves chemotherapeutic efficacy, inhibits metastasis, and increases antitumor T-cell responses. Tumor-educated macrophages also directly enhanced the tumor-initiating capacity of pancreatic tumor cells by activating the transcription factor STAT3, thereby facilitating macrophage-mediated suppression of CD8(+) T lymphocytes. Together, our findings show how targeting TAMs can effectively overcome therapeutic resistance mediated by TICs.
Conflict of interest statement
Figures




References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. - PubMed
-
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. - PubMed
-
- Pyonteck SM, Gadea BB, Wang HW, Gocheva V, Hunter KE, Tang LH, et al. Deficiency of the macrophage growth factor CSF-1 disrupts pancreatic neuroendocrine tumor development. Oncogene. 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

