Tumor suppressor function of the plasma glutathione peroxidase gpx3 in colitis-associated carcinoma
- PMID: 23221387
- PMCID: PMC3563732
- DOI: 10.1158/0008-5472.CAN-12-3150
Tumor suppressor function of the plasma glutathione peroxidase gpx3 in colitis-associated carcinoma
Abstract
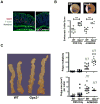
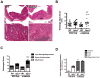
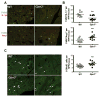
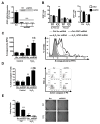
The glutathione peroxidases, a family of selenocysteine-containing redox enzymes, play pivotal roles in balancing the signaling, immunomodulatory, and deleterious effects of reactive oxygen species (ROS). The glutathione peroxidase GPX3 is the only extracellular member of this family, suggesting it may defend cells against ROS in the extracellular environment. Notably, GPX3 hypermethylation and underexpression occur commonly in prostate, gastric, cervical, thyroid, and colon cancers. We took a reverse genetics approach to investigate whether GPX3 would augment inflammatory colonic tumorigenesis, a process characterized by oxidative stress and inflammation, comparing Gpx3(-/-) mice in an established two-stage model of inflammatory colon carcinogenesis. Gpx3-deficient mice exhibited an increased tumor number, though not size, along with a higher degree of dysplasia. In addition, they exhibited increased inflammation with redistribution toward protumorigenic M2 macrophage subsets, increased proliferation, hyperactive WNT signaling, and increased DNA damage. To determine the impact of acute gene loss in an established colon cancer line, we silenced GPX3 in human Caco2 cells, resulting in increased ROS production, DNA damage and apoptosis in response to oxidative stress, combined with decreased contact-independent growth. Taken together, our results suggested an immunomodulatory role for GPX3 that limits the development of colitis-associated carcinoma.
Conflict of interest statement
There are no conflicts of interest to disclose
Figures


References
-
- Dincer Y, Erzin Y, Himmetoglu S, Gunes KN, Bal K, Akcay T. Oxidative DNA damage and antioxidant activity in patients with inflammatory bowel disease. Dig Dis Sci. 2007 Jul;52(7):1636–41. - PubMed
-
- Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004 Jul;287(1):G7–17. - PubMed
-
- Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990 Nov 1;323(18):1228–33. - PubMed
-
- Irons R, Carlson BA, Hatfield DL, Davis CD. Both selenoproteins and low molecular weight selenocompounds reduce colon cancer risk in mice with genetically impaired selenoprotein expression. J Nutr. 2006 May;136(5):1311–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50CA095103/CA/NCI NIH HHS/United States
- P50 CA095103/CA/NCI NIH HHS/United States
- AT004821-S1/AT/NCCIH NIH HHS/United States
- DK053620/DK/NIDDK NIH HHS/United States
- P01 CA116087/CA/NCI NIH HHS/United States
- I01 BX001453/BX/BLRD VA/United States
- I01 BX001426/BX/BLRD VA/United States
- P30DK058404/DK/NIDDK NIH HHS/United States
- P30CA068485/CA/NCI NIH HHS/United States
- F31 CA167920/CA/NCI NIH HHS/United States
- R01 DK053620/DK/NIDDK NIH HHS/United States
- 1F31CA167920/CA/NCI NIH HHS/United States
- DK080221/DK/NIDDK NIH HHS/United States
- R01 DK82813/DK/NIDDK NIH HHS/United States
- R01 AT004821/AT/NCCIH NIH HHS/United States
- AT004821/AT/NCCIH NIH HHS/United States
- K08 DK080221/DK/NIDDK NIH HHS/United States
- R01 DK082813/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

