Reduction of spinal sensory transmission by facilitation of 5-HT1B/D receptors in noninjured and spinal cord-injured humans
- PMID: 23221401
- PMCID: PMC3602943
- DOI: 10.1152/jn.00822.2012
Reduction of spinal sensory transmission by facilitation of 5-HT1B/D receptors in noninjured and spinal cord-injured humans
Abstract
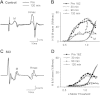
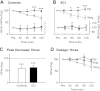
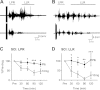
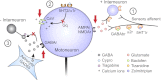
Activation of receptors by serotonin (5-HT1) and norepinephrine (α2) on primary afferent terminals and excitatory interneurons reduces transmission in spinal sensory pathways. Loss or reduction of descending sources of serotonin and norepinephrine after spinal cord injury (SCI) and the subsequent reduction of 5-HT1/α2 receptor activity contributes, in part, to the emergence of excessive motoneuron activation from sensory afferent pathways and the uncontrolled triggering of persistent inward currents that depolarize motoneurons during muscle spasms. We tested in a double-blind, placebo-controlled study whether facilitating 5-HT1B/D receptors with the agonist zolmitriptan reduces the sensory activation of motoneurons during an H-reflex in both noninjured control and spinal cord-injured participants. In both groups zolmitriptan, but not placebo, reduced the size of the maximum soleus H-reflex with a peak decrease to 59% (noninjured) and 62% (SCI) of predrug values. In SCI participants we also examined the effects of zolmitriptan on the cutaneomuscular reflex evoked in tibialis anterior from stimulation to the medial arch of the foot. Zolmitriptan, but not placebo, reduced the long-latency, polysynaptic component of the cutaneomuscular reflex (first 200 ms of reflex) by ∼50%. This ultimately reduced the triggering of the long-lasting component of the reflex (500 ms poststimulation to end of reflex) known to be mediated by persistent inward currents in the motoneuron. These results demonstrate that facilitation of 5-HT1B/D receptors reduces sensory transmission in both monosynaptic and polysynaptic reflex pathways to ultimately reduce long-lasting reflexes (spasms) after SCI.
Figures

Similar articles
-
Firing characteristics of deep dorsal horn neurons after acute spinal transection during administration of agonists for 5-HT1B/1D and NMDA receptors.J Neurophysiol. 2016 Oct 1;116(4):1644-1653. doi: 10.1152/jn.00198.2016. Epub 2016 Jul 13. J Neurophysiol. 2016. PMID: 27486104 Free PMC article.
-
Bursting interneurons in the deep dorsal horn develop increased excitability and sensitivity to serotonin after chronic spinal injury.J Neurophysiol. 2020 May 1;123(5):1657-1670. doi: 10.1152/jn.00701.2019. Epub 2020 Mar 25. J Neurophysiol. 2020. PMID: 32208883 Free PMC article.
-
Constitutively active 5-HT2/α1 receptors facilitate muscle spasms after human spinal cord injury.J Neurophysiol. 2013 Mar;109(6):1473-84. doi: 10.1152/jn.00821.2012. Epub 2012 Dec 5. J Neurophysiol. 2013. PMID: 23221402 Free PMC article.
-
[Electrophysiological assessment of reflex pathways involved in spasticity].Neurochirurgie. 2003 May;49(2-3 Pt 2):205-14. Neurochirurgie. 2003. PMID: 12746695 Review. French.
-
Modulation of the intrinsic properties of motoneurons by serotonin.Curr Pharm Des. 2013;19(24):4371-84. doi: 10.2174/13816128113199990341. Curr Pharm Des. 2013. PMID: 23360270 Review.
Cited by
-
Transcriptome Analysis Reveals the Effects of Troxerutin and Cerebroprotein Hydrolysate Injection on Injured Spinal Cords in Rats.Evid Based Complement Alternat Med. 2020 Aug 4;2020:3561235. doi: 10.1155/2020/3561235. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32831862 Free PMC article.
-
Selective effects of baclofen on use-dependent modulation of GABAB inhibition after tetraplegia.J Neurosci. 2013 Jul 31;33(31):12898-907. doi: 10.1523/JNEUROSCI.1552-13.2013. J Neurosci. 2013. PMID: 23904624 Free PMC article.
-
5-HT1D receptors inhibit the monosynaptic stretch reflex by modulating C-fiber activity.J Neurophysiol. 2019 May 1;121(5):1591-1608. doi: 10.1152/jn.00805.2018. Epub 2019 Jan 9. J Neurophysiol. 2019. PMID: 30625007 Free PMC article.
-
Enhancing KCC2 activity decreases hyperreflexia and spasticity after chronic spinal cord injury.Exp Neurol. 2021 Apr;338:113605. doi: 10.1016/j.expneurol.2021.113605. Epub 2021 Jan 13. Exp Neurol. 2021. PMID: 33453210 Free PMC article.
-
Neuromodulatory Inputs to Motoneurons Contribute to the Loss of Independent Joint Control in Chronic Moderate to Severe Hemiparetic Stroke.Front Neurol. 2018 Jun 21;9:470. doi: 10.3389/fneur.2018.00470. eCollection 2018. Front Neurol. 2018. PMID: 29977224 Free PMC article.
References
-
- Anden NE, Haeggendal J, Magnusson T, Rosengren E. The time course of the disappearance of noradrenaline and 5-hydroxytryptamine in the spinal cord after transection. Acta Physiol Scand 62: 115–118, 1964 - PubMed
-
- Antri M, Barthe JY, Mouffle C, Orsal D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci Lett 384: 162–7, 2005 - PubMed
-
- Baker LL, Chandler SH. Characterization of postsynaptic potentials evoked by sural nerve stimulation in hindlimb motoneurons from acute and chronic spinal cats. Brain Res 420: 340–350, 1987 - PubMed
-
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol 86: 1955–1971, 2001a - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

