Identification and structural characterization of a broadly neutralizing antibody targeting a novel conserved epitope on the influenza virus H5N1 hemagglutinin
- PMID: 23221567
- PMCID: PMC3571485
- DOI: 10.1128/JVI.02344-12
Identification and structural characterization of a broadly neutralizing antibody targeting a novel conserved epitope on the influenza virus H5N1 hemagglutinin
Abstract
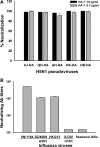
The unabated circulation of the highly pathogenic avian influenza A virus/H5N1 continues to be a serious threat to public health worldwide. Because of the high frequency of naturally occurring mutations, the emergence of H5N1 variants with high virulence has raised great concerns about the potential transmissibility of the virus in humans. Recent studies have shown that laboratory-mutated or reassortant H5N1 viruses could be efficiently transmitted among mammals, particularly ferrets, the best animal model for humans. Thus, it is critical to establish effective strategies to combat future H5N1 pandemics. In this study, we identified a broadly neutralizing monoclonal antibody (MAb), HA-7, that potently neutralized all tested strains of H5N1 covering clades 0, 1, 2.2, 2.3.4, and 2.3.2.1 and completely protected mice against lethal challenges of H5N1 viruses from clades 1 and 2.3.4. HA-7 specifically targeted the globular head of the H5N1 virus hemagglutinin (HA). Using electron microscopy technology with three-dimensional reconstruction (3D-EM), we discovered that HA-7 bound to a novel and highly conserved conformational epitope that was centered on residues 81 to 83 and 117 to 122 of HA1 (H5 numbering). We further demonstrated that HA-7 inhibited viral entry during postattachment events but not at the receptor-binding step, which is fully consistent with the 3D-EM result. Taken together, we propose that HA-7 could be humanized as an effective passive immunotherapeutic agent for antiviral stockpiling for future influenza pandemics caused by emerging unpredictable H5N1 strains. Our study also provides a sound foundation for the rational design of vaccines capable of inducing broad-spectrum immunity against H5N1.
Figures





Similar articles
-
An antibody against a novel and conserved epitope in the hemagglutinin 1 subunit neutralizes numerous H5N1 influenza viruses.J Virol. 2010 Aug;84(16):8275-86. doi: 10.1128/JVI.02593-09. Epub 2010 Jun 2. J Virol. 2010. PMID: 20519402 Free PMC article.
-
A novel humanized antibody neutralizes H5N1 influenza virus via two different mechanisms.J Virol. 2015 Apr;89(7):3712-22. doi: 10.1128/JVI.03014-14. Epub 2015 Jan 21. J Virol. 2015. PMID: 25609802 Free PMC article.
-
A human antibody recognizing a conserved epitope of H5 hemagglutinin broadly neutralizes highly pathogenic avian influenza H5N1 viruses.J Virol. 2012 Mar;86(6):2978-89. doi: 10.1128/JVI.06665-11. Epub 2012 Jan 11. J Virol. 2012. PMID: 22238297 Free PMC article.
-
The antigenic architecture of the hemagglutinin of influenza H5N1 viruses.Mol Immunol. 2013 Dec;56(4):705-19. doi: 10.1016/j.molimm.2013.07.010. Epub 2013 Aug 7. Mol Immunol. 2013. PMID: 23933511 Review.
-
Broadly neutralizing antibodies against influenza viruses.Antiviral Res. 2013 Jun;98(3):476-83. doi: 10.1016/j.antiviral.2013.03.021. Epub 2013 Apr 9. Antiviral Res. 2013. PMID: 23583287 Free PMC article. Review.
Cited by
-
Asprellcosides B of Ilex asprella Inhibits Influenza A Virus Infection by Blocking the Hemagglutinin- Mediated Membrane Fusion.Front Microbiol. 2019 Jan 23;9:3325. doi: 10.3389/fmicb.2018.03325. eCollection 2018. Front Microbiol. 2019. PMID: 30728818 Free PMC article.
-
Chemically Modified Bovine β-Lactoglobulin as a Broad-Spectrum Influenza Virus Entry Inhibitor with the Potential to Combat Influenza Outbreaks.Viruses. 2022 Sep 16;14(9):2055. doi: 10.3390/v14092055. Viruses. 2022. PMID: 36146861 Free PMC article.
-
Inhibition of influenza A virus and SARS-CoV-2 infection or co-infection by griffithsin and griffithsin-based bivalent entry inhibitor.mBio. 2024 May 8;15(5):e0074124. doi: 10.1128/mbio.00741-24. Epub 2024 Apr 9. mBio. 2024. PMID: 38587427 Free PMC article.
-
A novel hemagglutinin protein produced in bacteria protects chickens against H5N1 highly pathogenic avian influenza viruses by inducing H5 subtype-specific neutralizing antibodies.PLoS One. 2017 Feb 17;12(2):e0172008. doi: 10.1371/journal.pone.0172008. eCollection 2017. PLoS One. 2017. PMID: 28212428 Free PMC article.
-
Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry.Viruses. 2015 Dec 25;8(1):6. doi: 10.3390/v8010006. Viruses. 2015. PMID: 26712783 Free PMC article.
References
-
- Fouchier RA, Garcia-Sastre A, Kawaoka Y. 2012. Pause on avian flu transmission studies. Nature 481:443 doi:10.1038/481443a - DOI - PubMed
-
- Fouchier RA, Herfst S, Osterhaus AD. 2012. Public health and biosecurity. Restricted data on influenza H5N1 virus transmission. Science 335:662–663 - PubMed
-
- Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, Li C, Kawakami E, Yamada S, Kiso M, Suzuki Y, Maher EA, Neumann G, Kawaoka Y. 2012. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486:420–428 - PMC - PubMed
-
- Berns KI, Casadevall A, Cohen ML, Ehrlich SA, Enquist LW, Fitch JP, Franz DR, Fraser-Liggett CM, Grant CM, Imperiale MJ, Kanabrocki J, Keim PS, Lemon SM, Levy SB, Lumpkin JR, Miller JF, Murch R, Nance ME, Osterholm MT, Relman DA, Roth JA, Vidaver AK. 2012. Policy: adaptations of avian flu virus are a cause for concern. Nature 482:153–154 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

