Human mitochondrial RNA decay mediated by PNPase-hSuv3 complex takes place in distinct foci
- PMID: 23221631
- PMCID: PMC3553951
- DOI: 10.1093/nar/gks1130
Human mitochondrial RNA decay mediated by PNPase-hSuv3 complex takes place in distinct foci
Abstract
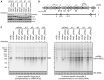
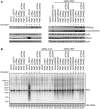
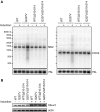
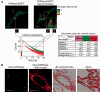
RNA decay is usually mediated by protein complexes and can occur in specific foci such as P-bodies in the cytoplasm of eukaryotes. In human mitochondria nothing is known about the spatial organization of the RNA decay machinery, and the ribonuclease responsible for RNA degradation has not been identified. We demonstrate that silencing of human polynucleotide phosphorylase (PNPase) causes accumulation of RNA decay intermediates and increases the half-life of mitochondrial transcripts. A combination of fluorescence lifetime imaging microscopy with Förster resonance energy transfer and bimolecular fluorescence complementation (BiFC) experiments prove that PNPase and hSuv3 helicase (Suv3, hSuv3p and SUPV3L1) form the RNA-degrading complex in vivo in human mitochondria. This complex, referred to as the degradosome, is formed only in specific foci (named D-foci), which co-localize with mitochondrial RNA and nucleoids. Notably, interaction between PNPase and hSuv3 is essential for efficient mitochondrial RNA degradation. This provides indirect evidence that degradosome-dependent mitochondrial RNA decay takes place in foci.
Figures





References
-
- Carpousis AJ. The RNA degradosome of Escherichia coli: an mRNA-degrading machine assembled on RNase E. Annu. Rev. Microbiol. 2007;61:71–87. - PubMed
-
- Dziembowski A, Piwowarski J, Hoser R, Minczuk M, Dmochowska A, Siep M, van der Spek H, Grivell L, Stepien PP. The yeast mitochondrial degradosome. Its composition, interplay between RNA helicase and RNase activities and the role in mitochondrial RNA metabolism. J. Biol. Chem. 2003;278:1603–1611. - PubMed
-
- Lykke-Andersen S, Tomecki R, Jensen TH, Dziembowski A. The eukaryotic RNA exosome: same scaffold but variable catalytic subunits. RNA Biol. 2011;8:61–66. - PubMed
-
- Chlebowski A, Tomecki R, Lopez ME, Seraphin B, Dziembowski A. Catalytic properties of the eukaryotic exosome. Adv. Exp. Med. Biol. 2011;702:63–78. - PubMed
-
- Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol. Cell. 2007;25:635–646. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

