Changes in bacterial growth rate govern expression of the Borrelia burgdorferi OspC and Erp infection-associated surface proteins
- PMID: 23222718
- PMCID: PMC3562092
- DOI: 10.1128/JB.01956-12
Changes in bacterial growth rate govern expression of the Borrelia burgdorferi OspC and Erp infection-associated surface proteins
Erratum in
-
Correction for Jutras et al., "Changes in Bacterial Growth Rate Govern Expression of the Borrelia burgdorferi OspC and Erp Infection-Associated Surface Proteins".J Bacteriol. 2017 Sep 5;199(19):e00386-17. doi: 10.1128/JB.00386-17. Print 2017 Oct 1. J Bacteriol. 2017. PMID: 28874456 Free PMC article. No abstract available.
Abstract
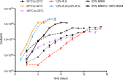
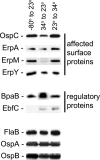
The Lyme disease spirochete controls production of its OspC and Erp outer surface proteins, repressing protein synthesis during colonization of vector ticks but increasing expression when those ticks feed on vertebrate hosts. Early studies found that the synthesis of OspC and Erps can be stimulated in culture by shifting the temperature from 23°C to 34°C, leading to a hypothesis that Borrelia burgdorferi senses environmental temperature to determine its location in the tick-mammal infectious cycle. However, borreliae cultured at 34°C divide several times faster than do those cultured at 23°C. We developed methods that disassociate bacterial growth rate and temperature, allowing a separate evaluation of each factor's impacts on B. burgdorferi gene and protein expression. Altogether, the data support a new paradigm that B. burgdorferi actually responds to changes in its own replication rate, not temperature per se, as the impetus to increase the expression of the OspC and Erp infection-associated proteins.
Figures


Similar articles
-
Transcriptional regulation of the ospAB and ospC promoters from Borrelia burgdorferi.Mol Microbiol. 2003 Jun;48(6):1665-77. doi: 10.1046/j.1365-2958.2003.03537.x. Mol Microbiol. 2003. PMID: 12791146
-
OspC is potent plasminogen receptor on surface of Borrelia burgdorferi.J Biol Chem. 2012 May 11;287(20):16860-8. doi: 10.1074/jbc.M111.290775. Epub 2012 Mar 20. J Biol Chem. 2012. PMID: 22433849 Free PMC article.
-
Regulation of the virulence determinant OspC by bbd18 on linear plasmid lp17 of Borrelia burgdorferi.J Bacteriol. 2011 Oct;193(19):5365-73. doi: 10.1128/JB.01496-10. Epub 2011 Jul 22. J Bacteriol. 2011. PMID: 21784941 Free PMC article.
-
Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi.Biochem Soc Trans. 2003 Feb;31(Pt 1):108-12. doi: 10.1042/bst0310108. Biochem Soc Trans. 2003. PMID: 12546665 Review.
-
Evolving models of Lyme disease spirochete gene regulation.Wien Klin Wochenschr. 2006 Nov;118(21-22):643-52. doi: 10.1007/s00508-006-0690-2. Wien Klin Wochenschr. 2006. PMID: 17160602 Review.
Cited by
-
RNA-Seq of Borrelia burgdorferi in Multiple Phases of Growth Reveals Insights into the Dynamics of Gene Expression, Transcriptome Architecture, and Noncoding RNAs.PLoS One. 2016 Oct 5;11(10):e0164165. doi: 10.1371/journal.pone.0164165. eCollection 2016. PLoS One. 2016. PMID: 27706236 Free PMC article.
-
Coupled induction of prophage and virulence factors during tick transmission of the Lyme disease spirochete.Nat Commun. 2023 Jan 13;14(1):198. doi: 10.1038/s41467-023-35897-3. Nat Commun. 2023. PMID: 36639656 Free PMC article.
-
Borrelia burgdorferi DnaA and the Nucleoid-Associated Protein EbfC Coordinate Expression of the dnaX-ebfC Operon.J Bacteriol. 2023 Jan 26;205(1):e0039622. doi: 10.1128/jb.00396-22. Epub 2022 Dec 19. J Bacteriol. 2023. PMID: 36533911 Free PMC article.
-
Inactivation of genes for antigenic variation in the relapsing fever spirochete Borrelia hermsii reduces infectivity in mice and transmission by ticks.PLoS Pathog. 2014 Apr 3;10(4):e1004056. doi: 10.1371/journal.ppat.1004056. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24699793 Free PMC article.
-
The Lyme disease spirochete's BpuR DNA/RNA-binding protein is differentially expressed during the mammal-tick infectious cycle, which affects translation of the SodA superoxide dismutase.Mol Microbiol. 2019 Sep;112(3):973-991. doi: 10.1111/mmi.14336. Epub 2019 Jul 7. Mol Microbiol. 2019. PMID: 31240776 Free PMC article.
References
-
- Stevenson B, von Lackum K, Riley SP, Cooley AE, Woodman ME, Bykowski T. 2006. Evolving models of Lyme disease spirochete gene regulation. Wien. Klin. Wochenschr. 118: 643–652 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

