Cytotoxic effector function of CD4-independent, CD8(+) T cells is mediated by TNF-α/TNFR
- PMID: 23222736
- PMCID: PMC3522862
- DOI: 10.1097/TP.0b013e318270f3c0
Cytotoxic effector function of CD4-independent, CD8(+) T cells is mediated by TNF-α/TNFR
Abstract
Background: Liver parenchymal cell allografts initiate both CD4-dependent and CD4-independent, CD8(+) T cell-mediated acute rejection pathways. The magnitude of allospecific CD8(+) T cell in vivo cytotoxic effector function is maximal when primed in the presence of CD4(+) T cells. The current studies were conducted to determine if and how CD4(+) T cells might influence cytotoxic effector mechanisms.
Methods: Mice were transplanted with allogeneic hepatocytes. In vivo cytotoxicity assays and various gene-deficient recipient mice and target cells were used to determine the development of Fas-, TNF-α-, and perforin-dependent cytotoxic effector mechanisms after transplantation.
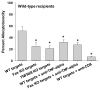
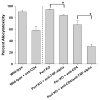
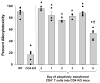
Results: CD8(+) T cells maturing in CD4-sufficient hepatocyte recipients develop multiple (Fas-, TNF-α-, and perforin-mediated) cytotoxic mechanisms. However, CD8(+) T cells, maturing in the absence of CD4(+) T cells, mediate cytotoxicity and transplant rejection that is exclusively TNF-α/TNFR-dependent. To determine the kinetics of CD4-mediated help, CD4(+) T cells were adoptively transferred into CD4-deficient mice at various times posttransplant. The maximal influence of CD4(+) T cells on the magnitude of CD8-mediated in vivo allocytotoxicityf occurs within 48 hours.
Conclusion: The implication of these studies is that interference of CD4(+) T cell function by disease or immunotherapy will have downstream consequences on both the magnitude of allocytotoxicity as well as the cytotoxic effector mechanisms used by allospecific CD8(+) cytolytic T cells.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose.
Figures



References
-
- Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171 (1):27. - PubMed
-
- Halloran PF, Urmson J, Ramassar V, et al. Lesions of T-cell-mediated kidney allograft rejection in mice do not require perforin or granzymes A and B. Am J Transplant. 2004;4 (5):705. - PubMed
-
- O’Connell PJ, Pacheco-Silva A, Nickerson PW, et al. Unmodified pancreatic islet allograft rejection results in the preferential expression of certain T cell activation transcripts. J Immunol. 1993;150 (3):1093. - PubMed
-
- Han D, Xu X, Baidal D, et al. Assessment of cytotoxic lymphocyte gene expression in the peripheral blood of human islet allograft recipients: elevation precedes clinical evidence of rejection. Diabetes. 2004;53 (9):2281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

