MicroRNA-7 regulates the mTOR pathway and proliferation in adult pancreatic β-cells
- PMID: 23223022
- PMCID: PMC3581216
- DOI: 10.2337/db12-0451
MicroRNA-7 regulates the mTOR pathway and proliferation in adult pancreatic β-cells
Abstract
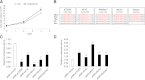
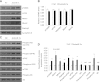
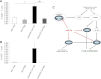
Elucidating the mechanism underlying the poor proliferative capacity of adult pancreatic β-cells is critical to regenerative therapeutic approaches for diabetes. Here, we show that the microRNA (miR)-7/7ab family member miR-7a is enriched in mouse adult pancreatic islets compared with miR-7b. Remarkably, miR-7a targets five components of the mTOR signaling pathway. Further, inhibition of miR-7a activates mTOR signaling and promotes adult β-cell replication in mouse primary islets, which can be reversed by the treatment with a well-known mTOR inhibitor, rapamycin. These data suggest that miR-7 acts as a brake on adult β-cell proliferation. Most importantly, this miR-7-mTOR proliferation axis is conserved in primary human β-cells, implicating miR-7 as a therapeutic target for diabetes.
Figures




Comment in
-
MicroRNA-7 control of β-cell replication.Diabetes. 2013 Mar;62(3):694-5. doi: 10.2337/db12-1518. Diabetes. 2013. PMID: 23431012 Free PMC article. No abstract available.
Similar articles
-
The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway.Peptides. 2013 Jan;39:71-9. doi: 10.1016/j.peptides.2012.10.006. Epub 2012 Oct 30. Peptides. 2013. PMID: 23116613
-
Mechanism of miR-122-5p regulating the activation of PI3K-Akt-mTOR signaling pathway on the cell proliferation and apoptosis of osteosarcoma cells through targeting TP53 gene.Eur Rev Med Pharmacol Sci. 2020 Dec;24(24):12655-12666. doi: 10.26355/eurrev_202012_24163. Eur Rev Med Pharmacol Sci. 2020. PMID: 33378012
-
EGF suppresses the expression of miR-124a in pancreatic β cell lines via ETS2 activation through the MEK and PI3K signaling pathways.Int J Biol Sci. 2019 Sep 7;15(12):2561-2575. doi: 10.7150/ijbs.34985. eCollection 2019. Int J Biol Sci. 2019. PMID: 31754329 Free PMC article.
-
mTOR: A double-edged sword for diabetes.J Leukoc Biol. 2019 Aug;106(2):385-395. doi: 10.1002/JLB.3MR0317-095RR. Epub 2018 Mar 26. J Leukoc Biol. 2019. PMID: 29578634 Review.
-
miRNAs in insulin resistance and diabetes-associated pancreatic cancer: the 'minute and miracle' molecule moving as a monitor in the 'genomic galaxy'.Curr Drug Targets. 2013 Sep;14(10):1110-7. doi: 10.2174/13894501113149990182. Curr Drug Targets. 2013. PMID: 23834149 Review.
Cited by
-
The circular RNA Cdr1as, via miR-7 and its targets, regulates insulin transcription and secretion in islet cells.Sci Rep. 2015 Jul 27;5:12453. doi: 10.1038/srep12453. Sci Rep. 2015. PMID: 26211738 Free PMC article.
-
Machine learning workflows identify a microRNA signature of insulin transcription in human tissues.iScience. 2021 Mar 31;24(4):102379. doi: 10.1016/j.isci.2021.102379. eCollection 2021 Apr 23. iScience. 2021. PMID: 33981968 Free PMC article.
-
Molecular Morbidity Score-Can MicroRNAs Assess the Burden of Disease?Int J Mol Sci. 2024 Jul 24;25(15):8042. doi: 10.3390/ijms25158042. Int J Mol Sci. 2024. PMID: 39125612 Free PMC article. Review.
-
The microRNA miR-7a-5p ameliorates ischemic brain damage by repressing α-synuclein.Sci Signal. 2018 Dec 11;11(560):eaat4285. doi: 10.1126/scisignal.aat4285. Sci Signal. 2018. PMID: 30538177 Free PMC article.
-
Menin is required for optimal processing of the microRNA let-7a.J Biol Chem. 2014 Apr 4;289(14):9902-8. doi: 10.1074/jbc.M113.520692. Epub 2014 Feb 21. J Biol Chem. 2014. PMID: 24563463 Free PMC article.
References
-
- Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of beta cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab 2007;3:758–768 - PubMed
-
- Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes 2005;54:2557–2567 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

