Persistent hyperalgesia in the cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and changes in dorsal root ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept
- PMID: 23223118
- PMCID: PMC3530135
- DOI: 10.1213/ANE.0b013e31826e1007
Persistent hyperalgesia in the cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and changes in dorsal root ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept
Abstract
Background: Painful neuropathy is a dose-limiting side effect in cancer chemotherapy. To characterize this phenomenon, we examined pain behavior and analgesic actions in a mouse model of cisplatin polyneuropathy.
Methods: Male C57BL/6 mice received intraperitoneal cisplatin or saline (2.3 mg/kg/d) every other day 6 times over 2 weeks for a total dose of 13.8 mg/kg. Thermal escape latencies, mechanical allodynia using von Frey hairs, and observation of behavior/morbidity and body weights were assessed. After onset of allodynia, we examined the actions of intraperitoneal gabapentin (100 mg/kg), etanercept (20 and 40 mg/kg), ketorolac (15 mg/kg), and morphine (1, 3, and 10 mg/kg). Additionally, using the conditioned place preference (CPP) paradigm, we examined the effects of gabapentin and ketorolac on the presumed pain state initiated by cisplatin. Additionally, we examined the spinal cord and dorsal root ganglia (DRG) of cisplatin-treated mice.
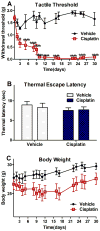
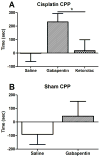
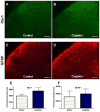
Results: Cisplatin, but not saline treatment, produced persistent hindpaw tactile allodynia, which persisted 46 days with no effect on thermal escape. Gabapentin and morphine, but neither etanercept nor ketorolac, produced a complete but transient (2-hour) reversal of the allodynia. Etanercept (40 mg/kg) pretreatment resulted in a delay in onset of mechanical allodynia. Using CPP, gabapentin, but not ketorolac, in cisplatin animals resulted in a significant preference for the drug-associated treatment compartment. There was no place preference in non-cisplatin-treated (nonallodynic) mice after gabapentin injection. Immunohistochemistry in cisplatin-treated mice showed no change in glial fibrillary acidic protein (astrocyte) or Iba1 (ionized calcium binding adaptor molecule 1) (microglia) activation states, but a significant increase in activated transcription factor 3 was observed in the DRG.
Conclusions: Cisplatintreated mice display allodynia and an activation of DRG activated transcription factor 3, which is paralleled by its effects on behavior in the CPP system, wherein gabapentin, but not ketorolac, in the presence of the cisplatin polyneuropathy, is positively rewarding, confirming that this neuropathy is an aversive (painful) state that is ameliorated by gabapentin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
The effect of gabapentin and ketorolac on allodynia and conditioned place preference in antibody-induced inflammation.Eur J Pain. 2016 Jul;20(6):917-25. doi: 10.1002/ejp.816. Epub 2015 Oct 30. Eur J Pain. 2016. PMID: 26517300 Free PMC article.
-
Spontaneous Chronic Pain After Experimental Thoracotomy Revealed by Conditioned Place Preference: Morphine Differentiates Tactile Evoked Pain From Spontaneous Pain.J Pain. 2015 Sep;16(9):903-12. doi: 10.1016/j.jpain.2015.06.006. Epub 2015 Jun 24. J Pain. 2015. PMID: 26116369 Free PMC article.
-
Differences in cisplatin-induced mechanical allodynia in male and female mice.Eur J Pain. 2015 Nov;19(10):1476-85. doi: 10.1002/ejp.679. Epub 2015 Feb 25. Eur J Pain. 2015. PMID: 25716290 Free PMC article.
-
Evaluation of the neonatal streptozotocin model of diabetes in rats: Evidence for a model of neuropathic pain.Pharmacol Rep. 2018 Apr;70(2):294-303. doi: 10.1016/j.pharep.2017.09.002. Epub 2017 Sep 14. Pharmacol Rep. 2018. PMID: 29477037 Free PMC article.
-
Efficacy of duloxetine, a potent and balanced serotonergic and noradrenergic reuptake inhibitor, in inflammatory and acute pain models in rodents.J Pharmacol Exp Ther. 2005 Feb;312(2):726-32. doi: 10.1124/jpet.104.075960. Epub 2004 Oct 19. J Pharmacol Exp Ther. 2005. PMID: 15494550
Cited by
-
Reward and motivation in pain and pain relief.Nat Neurosci. 2014 Oct;17(10):1304-12. doi: 10.1038/nn.3811. Epub 2014 Sep 25. Nat Neurosci. 2014. PMID: 25254980 Free PMC article. Review.
-
A New Rat Model of Cisplatin-induced Neuropathic Pain.Korean J Pain. 2015 Oct;28(4):236-43. doi: 10.3344/kjp.2015.28.4.236. Epub 2015 Oct 2. Korean J Pain. 2015. PMID: 26495078 Free PMC article.
-
HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy.Pain. 2017 Jun;158(6):1126-1137. doi: 10.1097/j.pain.0000000000000893. Pain. 2017. PMID: 28267067 Free PMC article.
-
Cancer Chemotherapy in Early Life Significantly Alters the Maturation of Pain Processing.Neuroscience. 2018 Sep 1;387:214-229. doi: 10.1016/j.neuroscience.2017.11.032. Epub 2017 Dec 2. Neuroscience. 2018. PMID: 29196027 Free PMC article.
-
Parabrachial Calca neurons drive nociplasticity.Cell Rep. 2024 Apr 23;43(4):114057. doi: 10.1016/j.celrep.2024.114057. Epub 2024 Apr 6. Cell Rep. 2024. PMID: 38583149 Free PMC article.
References
-
- Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. - PubMed
-
- Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44:1507–15. - PubMed
-
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–84. - PubMed
-
- Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291:1–9. - PubMed
-
- Kaley TJ, Deangelis LM. Therapy of chemotherapy-induced peripheral neuropathy. Br J Haematol. 2009;145:3–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

