The relation between perception and brain activity in gaze-evoked tinnitus
- PMID: 23223277
- PMCID: PMC6621667
- DOI: 10.1523/JNEUROSCI.2791-12.2012
The relation between perception and brain activity in gaze-evoked tinnitus
Abstract
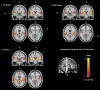
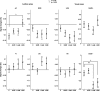
Tinnitus is a phantom sound percept that can be severely disabling. Its pathophysiology is poorly understood, partly due to the inability to objectively measure neural correlates of tinnitus. Gaze-evoked tinnitus (GET) is a rare form of tinnitus that may arise after vestibular schwannoma removal. Subjects typically describe tinnitus in the deaf ear on the side of the surgery that can be modulated by peripheral eye gaze. This phenomenon offers a unique opportunity to study the relation between tinnitus and brain activity. We used functional magnetic resonance imaging in humans to show that in normal-hearing control subjects, peripheral gaze results in inhibition of the auditory cortex, but no detectable response in the medial geniculate body (MGB) and inferior colliculus (IC). In patients with GET, peripheral gaze (1) reduced the cortical inhibition, (2) inhibited the MGB, and (3) activated the IC. Furthermore, increased tinnitus loudness is represented by increased activity in the cochlear nucleus (CN) and IC and reduced inhibition in the auditory cortex (AC). The increase of CN and IC activity with peripheral gaze is consistent with models of plastic reorganization in the brainstem following vestibular schwannoma removal. The activity decrease in the MGB and the reduced inhibition of the AC support a model that attributes tinnitus to a dysrhythmia of the thalamocortical loop, leading to hypometabolic theta activity in the MGB. Our data offer the first support of this loop hypothesis of tinnitus, independent of the initial experiments that led to its formulation.
Figures





References
-
- Baguley DM, Phillips J, Humphriss RL, Jones S, Axon PR, Moffat DA. The prevalence and onset of gaze modulation of tinnitus and increased sensitivity to noise after translabyrinthine vestibular schwannoma excision. Otol Neurotol. 2006;27:220–224. - PubMed
-
- Bateman N, Nikolopoulos TP, Robinson K, O'Donoghue GM. Impairments, disabilities, and handicaps after acoustic neuroma surgery. Clin Otolaryngol Allied Sci. 2000;25:62–65. - PubMed
-
- Baumgart F, Kaulisch T, Tempelmann C, Gaschler-Markefski B, Tegeler C, Schindler F, Stiller D, Scheich H. Electrodynamic headphones and woofers for application in magnetic resonance imaging scanners. Med Phys. 1998;25:2068–2070. - PubMed
-
- Biggs ND, Ramsden RT. Gaze-evoked tinnitus following acoustic neuroma resection: a de-afferentation plasticity phenomenon? Clin Otolaryngol Allied Sci. 2002;27:338–343. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
