Improving visual perception through neurofeedback
- PMID: 23223302
- PMCID: PMC3520425
- DOI: 10.1523/JNEUROSCI.6334-11.2012
Improving visual perception through neurofeedback
Abstract
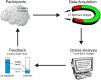
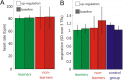
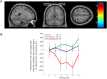
Perception depends on the interplay of ongoing spontaneous activity and stimulus-evoked activity in sensory cortices. This raises the possibility that training ongoing spontaneous activity alone might be sufficient for enhancing perceptual sensitivity. To test this, we trained human participants to control ongoing spontaneous activity in circumscribed regions of retinotopic visual cortex using real-time functional MRI-based neurofeedback. After training, we tested participants using a new and previously untrained visual detection task that was presented at the visual field location corresponding to the trained region of visual cortex. Perceptual sensitivity was significantly enhanced only when participants who had previously learned control over ongoing activity were now exercising control and only for that region of visual cortex. Our new approach allows us to non-invasively and non-pharmacologically manipulate regionally specific brain activity and thus provide "brain training" to deliver particular perceptual enhancements.
Figures








Comment in
-
Cognitive neuroscience: targeting neuroplasticity with neural decoding and biofeedback.Curr Biol. 2013 Mar 4;23(5):R210-2. doi: 10.1016/j.cub.2013.01.015. Curr Biol. 2013. PMID: 23473567
References
-
- Allen JJ, Harmon-Jones E, Cavender JH. Manipulation of frontal EEG asymmetry through biofeedback alters self-reported emotional responses and facial EMG. Psychophysiology. 2001;38:685–693. - PubMed
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: eplanation of the large variablity in evoked cortical responses. Science. 1996;273:1868–1871. - PubMed
-
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. - PubMed
-
- Birbaumer N, Ghanayim N, Hinterberger T, Iversen I, Kotchoubey B, Kübler A, Perelmouter J, Taub E, Flor H. A spelling device for the paralysed. Nature. 1999;398:297–298. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
