Increasing CRTC1 function in the dentate gyrus during memory formation or reactivation increases memory strength without compromising memory quality
- PMID: 23223304
- PMCID: PMC6621651
- DOI: 10.1523/JNEUROSCI.1419-12.2012
Increasing CRTC1 function in the dentate gyrus during memory formation or reactivation increases memory strength without compromising memory quality
Abstract
Memory stabilization following encoding (synaptic consolidation) or memory reactivation (reconsolidation) requires gene expression and protein synthesis (Dudai and Eisenberg, 2004; Tronson and Taylor, 2007; Nader and Einarsson, 2010; Alberini, 2011). Although consolidation and reconsolidation may be mediated by distinct molecular mechanisms (Lee et al., 2004), disrupting the function of the transcription factor CREB impairs both processes (Kida et al., 2002; Mamiya et al., 2009). Phosphorylation of CREB at Ser133 recruits CREB binding protein (CBP)/p300 coactivators to activate transcription (Chrivia et al., 1993; Parker et al., 1996). In addition to this well known mechanism, CREB regulated transcription coactivators (CRTCs), previously called transducers of regulated CREB (TORC) activity, stimulate CREB-mediated transcription, even in the absence of CREB phosphorylation. Recently, CRTC1 has been shown to undergo activity-dependent trafficking from synapses and dendrites to the nucleus in excitatory hippocampal neurons (Ch'ng et al., 2012). Despite being a powerful and specific coactivator of CREB, the role of CRTC in memory is virtually unexplored. To examine the effects of increasing CRTC levels, we used viral vectors to locally and acutely increase CRTC1 in the dorsal hippocampus dentate gyrus region of mice before training or memory reactivation in context fear conditioning. Overexpressing CRTC1 enhanced both memory consolidation and reconsolidation; CRTC1-mediated memory facilitation was context specific (did not generalize to nontrained context) and long lasting (observed after virally expressed CRTC1 dissipated). CREB overexpression produced strikingly similar effects. Therefore, increasing CRTC1 or CREB function is sufficient to enhance the strength of new, as well as established reactivated, memories without compromising memory quality.
Figures

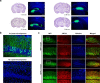
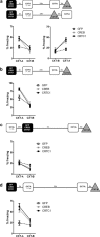
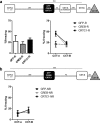
References
-
- Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
