Ethanol promotes clathrin adaptor-mediated endocytosis via the intracellular domain of δ-containing GABAA receptors
- PMID: 23223306
- PMCID: PMC3601804
- DOI: 10.1523/JNEUROSCI.2535-12.2012
Ethanol promotes clathrin adaptor-mediated endocytosis via the intracellular domain of δ-containing GABAA receptors
Abstract
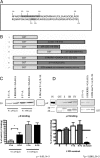
Pharmacological and genetic evidence reveals that GABA(A) receptor (GABA(A)-R) expression and localization are modulated in response to acute and chronic ethanol (EtOH) exposure. To determine molecular mechanisms of GABA(A)-R plasticity in response to in vivo acute EtOH, we measured early time changes in GABA(A)-R subunit localization. Single doses of EtOH (3 g/kg via i.p. injection in rats) produced decreases in surface levels of GABA(A)-R α4 and δ subunits at 5-15 min post-EtOH in hippocampus CA1 and dentate gyrus, verifying our earlier report (Liang et al., 2007). Here we also examined the β3 subunit and its phosphorylation state during internalization. β3 also was internalized during 5-15 min after EtOH exposure, while phosphorylation of β3 was increased, then decreased at later times, ruling out β3 dephosphorylation-dependent endocytosis. As early as 5 min post-EtOH, there is an initial increase in association between the δ subunits with clathrin adaptor proteins AP2-μ2 revealed by coimmunoprecipitation, followed by a decrease in association 15 min post-EtOH. In vitro studies using glutathione S-transferase fused to the δ subunit intracellular domain (ICD) show that two regions, one containing a classical YxxΦ motif and the other an atypical R/K-rich motif, directly and differentially bind to AP2-μ2, with the former YRSV exhibiting higher affinity. Mutating both regions in the δ-ICD abolishes μ2 binding, providing a possible mechanism that can explain the rapid downregulation of extrasynaptic α4βδ-GABA(A)-R following in vivo EtOH administration, in which the δ-ICD increases in affinity for clathrin AP2-μ2 leading to endocytosis.
Figures



Similar articles
-
Stabilization of GABA(A) receptors at endocytic zones is mediated by an AP2 binding motif within the GABA(A) receptor β3 subunit.J Neurosci. 2012 Feb 15;32(7):2485-98. doi: 10.1523/JNEUROSCI.1622-11.2011. J Neurosci. 2012. PMID: 22396422 Free PMC article.
-
Plasticity of GABAA receptors after ethanol pre-exposure in cultured hippocampal neurons.Mol Pharmacol. 2011 Mar;79(3):432-42. doi: 10.1124/mol.110.068650. Epub 2010 Dec 16. Mol Pharmacol. 2011. PMID: 21163967 Free PMC article.
-
Phospholipase C-related inactive protein is implicated in the constitutive internalization of GABAA receptors mediated by clathrin and AP2 adaptor complex.J Neurochem. 2007 May;101(4):898-905. doi: 10.1111/j.1471-4159.2006.04399.x. Epub 2007 Jan 24. J Neurochem. 2007. PMID: 17254016
-
The role of GABAAR phosphorylation in the construction of inhibitory synapses and the efficacy of neuronal inhibition.Biochem Soc Trans. 2009 Dec;37(Pt 6):1355-8. doi: 10.1042/BST0371355. Biochem Soc Trans. 2009. PMID: 19909275 Free PMC article. Review.
-
Regulation of GABA(A)-receptor surface expression with special reference to the involvement of GABARAP (GABA(A) receptor-associated protein) and PRIP (phospholipase C-related, but catalytically inactive protein).J Pharmacol Sci. 2007 Aug;104(4):285-92. doi: 10.1254/jphs.cp0070063. Epub 2007 Aug 10. J Pharmacol Sci. 2007. PMID: 17690529 Review.
Cited by
-
Targeting prefrontal cortex GABAergic microcircuits for the treatment of alcohol use disorder.Front Synaptic Neurosci. 2022 Aug 29;14:936911. doi: 10.3389/fnsyn.2022.936911. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 36105666 Free PMC article. Review.
-
Altered gamma oscillations during pregnancy through loss of δ subunit-containing GABA(A) receptors on parvalbumin interneurons.Front Neural Circuits. 2013 Sep 17;7:144. doi: 10.3389/fncir.2013.00144. eCollection 2013. Front Neural Circuits. 2013. PMID: 24062647 Free PMC article.
-
GABAA receptor membrane insertion rates are specified by their subunit composition.Mol Cell Neurosci. 2013 Sep;56:201-11. doi: 10.1016/j.mcn.2013.05.003. Epub 2013 May 25. Mol Cell Neurosci. 2013. PMID: 23714576 Free PMC article.
-
GABA type a receptor trafficking and the architecture of synaptic inhibition.Dev Neurobiol. 2018 Mar;78(3):238-270. doi: 10.1002/dneu.22536. Epub 2017 Sep 19. Dev Neurobiol. 2018. PMID: 28901728 Free PMC article. Review.
-
Ceramide is involved in alcohol-induced neural proliferation.Neural Regen Res. 2013 Aug 15;8(23):2178-89. doi: 10.3969/j.issn.1673-5374.2013.23.008. Neural Regen Res. 2013. PMID: 25206527 Free PMC article.
References
-
- Ali NJ, Olsen RW. Chronic benzodiazepine treatment of cells expressing recombinant GABA(A) receptors uncouples allosteric binding: studies on possible mechanisms. J Neurochem. 2001;79:1100–1108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R03 NS065725/NS/NINDS NIH HHS/United States
- R01 NS048045/NS/NINDS NIH HHS/United States
- R01 NS081986/NS/NINDS NIH HHS/United States
- R01 DA014137/DA/NIDA NIH HHS/United States
- P01 NS035985/NS/NINDS NIH HHS/United States
- R37 AA007680/AA/NIAAA NIH HHS/United States
- R01 AA007680/AA/NIAAA NIH HHS/United States
- R01 NS051195/NS/NINDS NIH HHS/United States
- NS35985/NS/NINDS NIH HHS/United States
- AA07680/AA/NIAAA NIH HHS/United States
- R01 NS056359/NS/NINDS NIH HHS/United States
- R01 NS047478/NS/NINDS NIH HHS/United States
- P01 GM058448/GM/NIGMS NIH HHS/United States
- P01 NS054900/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
