Rebalance of striatal NMDA/AMPA receptor ratio underlies the reduced emergence of dyskinesia during D2-like dopamine agonist treatment in experimental Parkinson's disease
- PMID: 23223310
- PMCID: PMC6621675
- DOI: 10.1523/JNEUROSCI.2664-12.2012
Rebalance of striatal NMDA/AMPA receptor ratio underlies the reduced emergence of dyskinesia during D2-like dopamine agonist treatment in experimental Parkinson's disease
Abstract
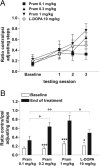
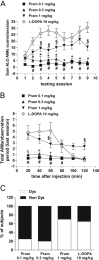
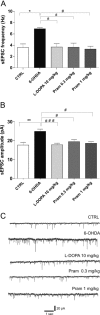
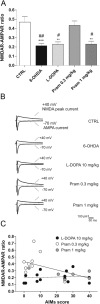
Dopamine replacement with levodopa (L-DOPA) represents the mainstay of Parkinson’s disease (PD) therapy. Nevertheless, this well established therapeutic intervention loses efficacy with the progression of the disease and patients develop invalidating side effects, known in their complex as L-DOPA-induced dyskinesia (LID). Unfortunately, existing therapies fail to prevent LID and very few drugs are available to lessen its severity, thus representing a major clinical problem inPDtreatment. D2-like receptor (D2R) agonists are a powerful clinical option as an alternative to L-DOPA, especially in the early stages of the disease, being associated to a reduced risk of dyskinesia development. D2R agonists also find considerable application in the advanced stages of PD, in conjunction with L-DOPA, which is used in this context at lower dosages, to delay the appearance and the extent of the motor complications. In advanced stages of PD, D2R agonists are often effective in delaying the appearance and the extent of motor complications. Despite the great attention paid to the family of D2R agonists, the main reasons underlying the reduced risk of dyskinesia have not yet been fully characterized. Here we show that the striatal NMDA/AMPAreceptor ratio and theAMPAreceptor subunit composition are altered in experimental parkinsonism in rats. Surprisingly, while L-DOPA fails to restore these critical synaptic alterations, chronic treatment with pramipexole is associated not only with a reduced risk of dyskinesia development but is also able to rebalance, in a dose-dependent fashion, the physiological synaptic parameters, thus providing new insights into the mechanisms of dyskinesia.
Figures

References
-
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. - PubMed
-
- Ahmed I, Bose SK, Pavese N, Ramlackhansingh A, Turkheimer F, Hotton G, Hammers A, Brooks DJ. Glutamate NMDA receptor dysregulation in Parkinson's disease with dyskinesias. Brain. 2011;134:979–986. - PubMed
-
- Bagetta V, Picconi B, Marinucci S, Sgobio C, Pendolino V, Ghiglieri V, Fusco FR, Giampà C, Calabresi P. Dopamine-dependent long-term depression is expressed in striatal spiny neurons of both direct and indirect pathways: implications for Parkinson's disease. J Neurosci. 2011;31:12513–12522. - PMC - PubMed
-
- Birkmayer W, Hornykiewicz O. The effect of l-3,4-dihydroxy phenylalanine (=DOPA) on akinesia in parkinsonism. Parkinsonism Relat Disord. 1998;4:59–60. - PubMed
