Regulation of cardiac ATP-sensitive potassium channel surface expression by calcium/calmodulin-dependent protein kinase II
- PMID: 23223335
- PMCID: PMC3548467
- DOI: 10.1074/jbc.M112.429548
Regulation of cardiac ATP-sensitive potassium channel surface expression by calcium/calmodulin-dependent protein kinase II
Abstract
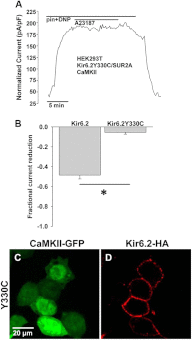
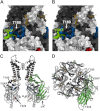
Cardiac ATP-sensitive potassium (K(ATP)) channels are key sensors and effectors of the metabolic status of cardiomyocytes. Alteration in their expression impacts their effectiveness in maintaining cellular energy homeostasis and resistance to injury. We sought to determine how activation of calcium/calmodulin-dependent protein kinase II (CaMKII), a central regulator of calcium signaling, translates into reduced membrane expression and current capacity of cardiac K(ATP) channels. We used real-time monitoring of K(ATP) channel current density, immunohistochemistry, and biotinylation studies in isolated hearts and cardiomyocytes from wild-type and transgenic mice as well as HEK cells expressing wild-type and mutant K(ATP) channel subunits to track the dynamics of K(ATP) channel surface expression. Results showed that activation of CaMKII triggered dynamin-dependent internalization of K(ATP) channels. This process required phosphorylation of threonine at 180 and 224 and an intact (330)YSKF(333) endocytosis motif of the K(ATP) channel Kir6.2 pore-forming subunit. A molecular model of the μ2 subunit of the endocytosis adaptor protein, AP2, complexed with Kir6.2 predicted that μ2 docks by interaction with (330)YSKF(333) and Thr-180 on one and Thr-224 on the adjacent Kir6.2 subunit. Phosphorylation of Thr-180 and Thr-224 would favor interactions with the corresponding arginine- and lysine-rich loops on μ2. We concluded that calcium-dependent activation of CaMKII results in phosphorylation of Kir6.2, which promotes endocytosis of cardiac K(ATP) channel subunits. This mechanism couples the surface expression of cardiac K(ATP) channels with calcium signaling and reveals new targets to improve cardiac energy efficiency and stress resistance.
Figures


References
-
- Weiss J. N., Venkatesh N. (1993) Metabolic regulation of cardiac ATP-sensitive K+ channels. Cardiovasc. Drugs Ther. 7, 499–505 - PubMed
-
- Noma A. (1983) ATP-regulated K+ channels in cardiac muscle. Nature 305, 147–148 - PubMed
-
- Aguilar-Bryan L., Bryan J. (1999) Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr. Rev. 20, 101–135 - PubMed
-
- Ashcroft F. M. (1988) Adenosine 5′-triphosphate-sensitive potassium channels. Annu. Rev. Neurosci. 11, 97–118 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL062494/HL/NHLBI NIH HHS/United States
- R01 HL096652/HL/NHLBI NIH HHS/United States
- R01 HL084583/HL/NHLBI NIH HHS/United States
- R01DK092412/DK/NIDDK NIH HHS/United States
- R01 HL113001/HL/NHLBI NIH HHS/United States
- R29 HL058812/HL/NHLBI NIH HHS/United States
- R01 HL070250/HL/NHLBI NIH HHS/United States
- K08 HL092286/HL/NHLBI NIH HHS/United States
- R01HL62494/HL/NHLBI NIH HHS/United States
- HL084583/HL/NHLBI NIH HHS/United States
- R01HL079031/HL/NHLBI NIH HHS/United States
- R01 HL079031/HL/NHLBI NIH HHS/United States
- K08 HL093368/HL/NHLBI NIH HHS/United States
- R01 HL083422/HL/NHLBI NIH HHS/United States
- HL083422/HL/NHLBI NIH HHS/United States
- R01 HL113089/HL/NHLBI NIH HHS/United States
- R01HL113001/HL/NHLBI NIH HHS/United States
- R01 DK092412/DK/NIDDK NIH HHS/United States
- R01HL113089/HL/NHLBI NIH HHS/United States
- G0802050/MRC_/Medical Research Council/United Kingdom
- I01 BX000718/BX/BLRD VA/United States
- HL093368/HL/NHLBI NIH HHS/United States
- R01HL70250/HL/NHLBI NIH HHS/United States
- R01 HL058812/HL/NHLBI NIH HHS/United States
- HL058812/HL/NHLBI NIH HHS/United States
- HL092286/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

