Treatment of young children with CNS-primitive neuroectodermal tumors/pineoblastomas in the prospective multicenter trial HIT 2000 using different chemotherapy regimens and radiotherapy
- PMID: 23223339
- PMCID: PMC3548584
- DOI: 10.1093/neuonc/nos292
Treatment of young children with CNS-primitive neuroectodermal tumors/pineoblastomas in the prospective multicenter trial HIT 2000 using different chemotherapy regimens and radiotherapy
Abstract
Background: Especially in young children, primitive neuroectodermal tumors of the central nervous system (CNS-PNET) and pineoblastomas are associated with an unfavorable outcome, and only a few prospective trials have been conducted thus far.
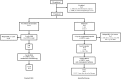
Methods: From January 2001 through January 2005, 17 eligible children aged <4 years with CNS-PNET not otherwise specified (n = 8), ependymoblastoma (n = 1), or pineoblastoma (n = 8) confirmed by central review were prospectively treated in the trial HIT 2000. In nonmetastatic disease (n = 11), up to 5 postoperative cycles of HIT-SKK systemic multiagent chemotherapy (8 months duration), followed by craniospinal radiotherapy (CSI), were given. In metastatic disease (M1-M3, n = 6), treatment consisted of a shorter induction chemotherapy (2-3 months) with carboplatin and etoposide, followed by tandem high-dose chemotherapy (HDCT) in case of good response to induction. During induction and HDCT, patients received intraventricular methotrexate. CSI was applied to all patients with poor response to induction or residual disease after HDCT and was optional for patients with residual disease before HDCT.
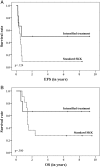
Results: Five-year event-free survival and overall survival rates ± standard error for all eligible patients were 24% ± 10% and 40% ± 12%, respectively (median follow-up of survivors: 8.3 years). Only one patient with nonmetastatic disease remained free of relapse/progressive disease during induction. Three of 6 patients with metastatic disease responded to induction and received tandem-HDCT, followed by preventive CSI, and remain in continuous complete remission.
Conclusions: Short intensive induction chemotherapy followed by tandem-HDCT in young children with CNS-PNET/pineoblastomas seems to be superior to the prolonged and less intensive induction regimen.
Figures



References
-
- Kaatsch P, Rickert CH, Kuhl J, Schuz J, Michaelis J. Population-based epidemiologic data on brain tumors in German children. Cancer. 2001;92(12):3155–3164. - PubMed
-
- Li MH, Bouffet E, Hawkins CE, Squire JA, Huang A. Molecular genetics of supratentorial primitive neuroectodermal tumors and pineoblastoma. Neurosurg Focus. 2005;19(5):E3. - PubMed
-
- Pfister S, Remke M, Toedt G, et al. Supratentorial primitive neuroectodermal tumors of the central nervous system frequently harbor deletions of the CDKN2A locus and other genomic aberrations distinct from medulloblastomas. Genes Chromosomes Cancer. 2007;46(9):839–851. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

