Murine anti-third-party central-memory CD8(+) T cells promote hematopoietic chimerism under mild conditioning: lymph-node sequestration and deletion of anti-donor T cells
- PMID: 23223359
- PMCID: PMC4467899
- DOI: 10.1182/blood-2012-07-441493
Murine anti-third-party central-memory CD8(+) T cells promote hematopoietic chimerism under mild conditioning: lymph-node sequestration and deletion of anti-donor T cells
Abstract
Transplantation of T cell-depleted BM (TDBM) under mild conditioning, associated with minimal toxicity and reduced risk of GVHD, offers an attractive therapeutic option for patients with nonmalignant hematologic disorders and can mediate immune tolerance to subsequent organ transplantation. However, overcoming TDBM rejection after reduced conditioning remains a challenge. Here, we address this barrier using donorderived central memory CD8(+) T cells (Tcms), directed against third-party antigens. Our results show that fully allogeneic or (hostXdonor)F1-Tcm, support donor chimerism (> 6 months) in sublethally irradiated (5.5Gy) mice, without GVHD symptoms. Chimerism under yet lower irradiation (4.5Gy) was achieved by combining Tcm with short-term administration of low-dose Rapamycin. Importantly, this chimerism resulted in successful donor skin acceptance, whereas third-party skin was rejected. Tracking of host anti-donor T cells (HADTCs), that mediate TDBMT rejection, in a novel bioluminescence-imaging model revealed that Tcms both induce accumulation and eradicate HADTCs in the LNs,concomitant with their elimination from other organs, including the BM. Further analysis with 2-photon microcopy revealed that Tcms form conjugates with HADTCs, resulting in decelerated and confined movement of HADTCs within the LNs in an antigen-specific manner. Thus, anti-third-party Tcms support TDBMT engraftment under reduced-conditioning through lymph-node sequestration and deletion of HADTCs, offering a novel and potentially safe approach for attaining stable hematopoietic chimerism.
Figures
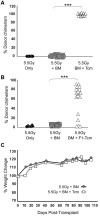

 ) or 5 × 106 CB6 F1 Tcm (H-2bd; ●). The indicated groups received subcutaneous injections of 0.5 mg/kg bw rapamycin during 5 days (days −1 to +4). Percentage of donor cells in peripheral blood, analyzed 90 days after transplant by FACS using anti-donor (H-2Kb) antibodies is presented. Data represents results of at least 2 independent experiments (*P < .05; ***P < .001).
) or 5 × 106 CB6 F1 Tcm (H-2bd; ●). The indicated groups received subcutaneous injections of 0.5 mg/kg bw rapamycin during 5 days (days −1 to +4). Percentage of donor cells in peripheral blood, analyzed 90 days after transplant by FACS using anti-donor (H-2Kb) antibodies is presented. Data represents results of at least 2 independent experiments (*P < .05; ***P < .001).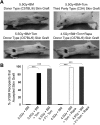

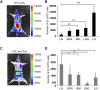
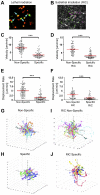
Comment in
-
Location, location, location: advancing veto cell therapies.Blood. 2013 Feb 14;121(7):1069-70. doi: 10.1182/blood-2012-12-472654. Blood. 2013. PMID: 23411733 No abstract available.
Similar articles
-
Induction of tolerance to bone marrow allografts by donor-derived host nonreactive ex vivo-induced central memory CD8 T cells.Blood. 2010 Mar 11;115(10):2095-104. doi: 10.1182/blood-2009-10-248716. Epub 2009 Dec 30. Blood. 2010. PMID: 20042725 Free PMC article.
-
Blockade of the CD40/CD154 pathway enhances T-cell-depleted allogeneic bone marrow engraftment under nonmyeloablative and irradiation-free conditioning therapy.Transplantation. 2003 Jul 15;76(1):216-24. doi: 10.1097/01.TP.0000069602.30162.A1. Transplantation. 2003. PMID: 12865813
-
The role of donor-derived veto cells in nonmyeloablative haploidentical HSCT.Bone Marrow Transplant. 2015 Jun;50 Suppl 2:S14-20. doi: 10.1038/bmt.2015.89. Bone Marrow Transplant. 2015. PMID: 26039201
-
Induction of transplantation tolerance in haploidenical transplantation under reduced intensity conditioning: the role of ex-vivo generated donor CD8+ T cells with central memory phenotype.Best Pract Res Clin Haematol. 2011 Sep;24(3):393-401. doi: 10.1016/j.beha.2011.05.007. Epub 2011 Jul 13. Best Pract Res Clin Haematol. 2011. PMID: 21925092 Review.
-
Protective conditioning against GVHD and graft rejection after combined organ and hematopoietic cell transplantation.Blood Cells Mol Dis. 2008 Jan-Feb;40(1):48-54. doi: 10.1016/j.bcmd.2007.06.019. Epub 2007 Sep 10. Blood Cells Mol Dis. 2008. PMID: 17827036 Review.
Cited by
-
Haploidentical SCT: the mechanisms underlying the crossing of HLA barriers.Bone Marrow Transplant. 2014 Jul;49(7):873-9. doi: 10.1038/bmt.2014.19. Epub 2014 Feb 24. Bone Marrow Transplant. 2014. PMID: 24566712 Review.
-
Allogeneic hematopoietic stem cell transplantation to cure sickle cell disease: A review.Front Med (Lausanne). 2023 Feb 23;10:1036939. doi: 10.3389/fmed.2023.1036939. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36910492 Free PMC article. Review.
-
Towards 'off-the-shelf' genetically modified T cells: prolonging functional engraftment in mice by CD8 veto T cells.Leukemia. 2018 Apr;32(4):1039-1041. doi: 10.1038/leu.2017.332. Epub 2017 Nov 20. Leukemia. 2018. PMID: 29151584 No abstract available.
-
Immune tolerance induction by nonmyeloablative haploidentical HSCT combining T-cell depletion and posttransplant cyclophosphamide.Blood Adv. 2017 Oct 30;1(24):2166-2175. doi: 10.1182/bloodadvances.2017009423. eCollection 2017 Nov 14. Blood Adv. 2017. PMID: 29296864 Free PMC article.
-
Correction of T-Cell Repertoire and Autoimmune Diabetes in NOD Mice by Non-myeloablative T-Cell Depleted Allogeneic HSCT.Stem Cells Transl Med. 2023 May 15;12(5):281-292. doi: 10.1093/stcltm/szad021. Stem Cells Transl Med. 2023. PMID: 37184893 Free PMC article.
References
-
- Reisner Y, Hagin D, Martelli MF. Haploidentical hematopoietic transplantation: current status and future perspectives. Blood. 2011;118(23):6006–6017. - PubMed
-
- Ophir E, Reisner Y. Induction of tolerance in organ recipients by hematopoietic stem cell transplantation. Int Immunopharmacol. 2009;9(6):694–700. - PubMed
-
- Sykes M, Sachs DH. Mixed allogeneic chimerism as an approach to transplantation tolerance. Immunol Today. 1988;9(1):23–27. - PubMed
-
- Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. J Immunol. 1994;153(3):1087–1098. - PubMed
-
- Gur H, Krauthgamer R, Berrebi A, et al. Tolerance induction by megadose hematopoietic progenitor cells: expansion of veto cells by short-term culture of purified human CD34(+) cells. Blood. 2002;99(11):4174–4181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

