Microfluidic Western blotting
- PMID: 23223527
- PMCID: PMC3535594
- DOI: 10.1073/pnas.1207754110
Microfluidic Western blotting
Abstract
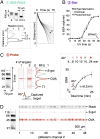
Rapid, quantitative Western blotting is a long-sought bioanalytical goal in the life sciences. To this end, we describe a Western blotting assay conducted in a single glass microchannel under purely electronic control. The μWestern blot is comprised of multiple steps: sample enrichment, protein sizing, protein immobilization (blotting), and in situ antibody probing. To validate the microfluidic assay, we apply the μWestern blot to analyses of human sera (HIV immunoreactivity) and cell lysate (NFκB). Analytical performance advances are achieved, including: short durations of 10-60 min, multiplexed analyte detection, mass sensitivity at the femtogram level, high-sensitivity 50-pM detection limits, and quantitation capability over a 3.6-log dynamic range. Performance gains are attributed to favorable transport and reaction conditions on the microscale. The multistep assay design relies on a photopatternable (blue light) and photoreactive (UV light) polyacrylamide gel. This hydrophilic polymer constitutes both a separation matrix for protein sizing and, after brief UV exposure, a protein immobilization scaffold for subsequent antibody probing of immobilized protein bands. We observe protein capture efficiencies exceeding 75% under sizing conditions. This compact microfluidic design supports demonstration of a 48-plex μWestern blot in a standard microscope slide form factor. Taken together, the μWestern blot establishes a foundation for rapid, targeted proteomics by merging exceptional specificity with the throughput advantages of multiplexing, as is relevant to a broad range of biological inquiry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Microfluidic Western blotting of low-molecular-mass proteins.Anal Chem. 2014 Nov 4;86(21):10625-32. doi: 10.1021/ac5024588. Epub 2014 Oct 15. Anal Chem. 2014. PMID: 25268977 Free PMC article.
-
Microfluidic integration for automated targeted proteomic assays.Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):5972-7. doi: 10.1073/pnas.1108617109. Epub 2012 Apr 2. Proc Natl Acad Sci U S A. 2012. PMID: 22474344 Free PMC article.
-
Western blotting using microfluidics.Methods Mol Biol. 2015;1312:469-72. doi: 10.1007/978-1-4939-2694-7_48. Methods Mol Biol. 2015. PMID: 26044029
-
In Situ Single-Cell Western Blot on Adherent Cell Culture.Angew Chem Int Ed Engl. 2019 Sep 23;58(39):13929-13934. doi: 10.1002/anie.201906920. Epub 2019 Aug 21. Angew Chem Int Ed Engl. 2019. PMID: 31390130 Free PMC article. Review.
-
An overview of Western blotting for determining antibody specificities for immunohistochemistry.Methods Mol Biol. 2011;717:55-67. doi: 10.1007/978-1-61779-024-9_3. Methods Mol Biol. 2011. PMID: 21370024 Review.
Cited by
-
High-throughput screening of high lactic acid-producing Bacillus coagulans by droplet microfluidic based flow cytometry with fluorescence activated cell sorting.RSC Adv. 2019 Feb 5;9(8):4507-4513. doi: 10.1039/c8ra09684h. eCollection 2019 Jan 30. RSC Adv. 2019. PMID: 35520173 Free PMC article.
-
Highly parallel production of designer organoids by mosaic patterning of progenitors.Cell Syst. 2024 Jul 17;15(7):649-661.e9. doi: 10.1016/j.cels.2024.06.004. Epub 2024 Jul 8. Cell Syst. 2024. PMID: 38981488 Free PMC article.
-
Microfluidic Devices for HIV Diagnosis and Monitoring at Point-of-Care (POC) Settings.Biosensors (Basel). 2022 Nov 1;12(11):949. doi: 10.3390/bios12110949. Biosensors (Basel). 2022. PMID: 36354458 Free PMC article. Review.
-
A lateral electrophoretic flow diagnostic assay.Lab Chip. 2015 Mar 21;15(6):1488-96. doi: 10.1039/c4lc01370k. Lab Chip. 2015. PMID: 25608872 Free PMC article.
-
Protein Microarrays with Novel Microfluidic Methods: Current Advances.Microarrays (Basel). 2014 Jul 1;3(3):180-202. doi: 10.3390/microarrays3030180. Microarrays (Basel). 2014. PMID: 27600343 Free PMC article. Review.
References
-
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. - PubMed
-
- Kurien BT, Scofield RH. Protein Blotting and Detection: Methods and Protocols. New York: Springer; 2009.
-
- Southern E. Southern blotting. Nat Protoc. 2006;1(2):518–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

